
- Автоматизация
- Антропология
- Археология
- Архитектура
- Биология
- Ботаника
- Бухгалтерия
- Военная наука
- Генетика
- География
- Геология
- Демография
- Деревообработка
- Журналистика
- Зоология
- Изобретательство
- Информатика
- Искусство
- История
- Кинематография
- Компьютеризация
- Косметика
- Кулинария
- Культура
- Лексикология
- Лингвистика
- Литература
- Логика
- Маркетинг
- Математика
- Материаловедение
- Медицина
- Менеджмент
- Металлургия
- Метрология
- Механика
- Музыка
- Науковедение
- Образование
- Охрана Труда
- Педагогика
- Полиграфия
- Политология
- Право
- Предпринимательство
- Приборостроение
- Программирование
- Производство
- Промышленность
- Психология
- Радиосвязь
- Религия
- Риторика
- Социология
- Спорт
- Стандартизация
- Статистика
- Строительство
- Технологии
- Торговля
- Транспорт
- Фармакология
- Физика
- Физиология
- Философия
- Финансы
- Химия
- Хозяйство
- Черчение
- Экология
- Экономика
- Электроника
- Электротехника
- Энергетика
1.3 Assessed Homework Mark Scheme 2008
1. 3 Assessed Homework Mark Scheme 2008
1. (a) (i) Covalent (1)
(ii) Co-ordinate (1) (or dative)
(iii) Both / two / pair electrons come from nitrogen (1)
(iv) 4 bonding / electron pairs (1)
repel equally (1)
OR are identical
as far apart as possible (1)
OR to position of minimum repulsion
tetrahedron (1) 7
(b) Power (or ability) of an element / atom to attract electron pair / electrons/
an electron/electron density (1)
in a covalent bond (1)
Allow attract from, withdraw in, do not allow remove from, withdraw from.
2
(c) (i) Electron deficient (1)
Or small, slight, partial positive charge
(ii) H < N (1) 2
[11]
2. (a) (i) Electronegativity (difference) or suitable description (1)
Accept F and Cl are highly electronegative
Not both atoms are highly electronegative
(ii) HF = hydrogen bonding (1)
HCl = (permanent) dipole-dipole bonding or even van de Waals’ (1)
Hydrogen bonding stronger / is the strongest IMF (1) 4
Accept a statement that HF must have the stronger IMF, even if no IMFs identified
The explanation must be based on intermolecular forces/attractions
Note: if the explanation is clearlyintramolecular = CE
(b) Electron pair or lone pair donated (1)
Do not accept ‘donation of electrons’
From chloride ion to Al or AlCl3 (1) 2
M1 can be earned by a general explanation of coordinate bonding, even if the electron pair is said to come from Al. The second mark, M2, is for this specific bond
Ignore missing charge
(c) 4
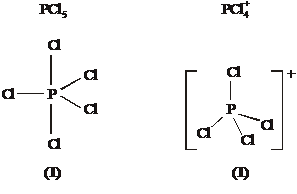
PCl5 shown as trigonal bipyramid PCl4+ shown as tetrahedral
[Look for: ONE solid linear Cl-P-Cl bond] NO solid linear Cl-P-Cl bonds]
Bond Angle(s) 90° and 120° (1) Bond angle(s) 109 or 109. 5° (1)
[10]
3. (a) 4 LiH + AlCl3 à LiAlH4 + 3 LiCl 1
(b) H – = 1s2 or 1s2 1
(c) Tetrahedral or diagram 1
(Not distorted tetrahedral)
(Equal) repulsion 1
between four bonding pairs / bonds 1
(Not repulsion between H atoms loses M2 and M3)
(Not ‘separate as far as possible’)
(‘4’ may be inferred from a correct diagram)
(d) Dative (covalent) or coordinate 1
Lone pair or non-bonding pair of electron or both e– 1
QoL Donated from H– to Al or shared between H and Al 1
(tied to M2)
(Not ‘from H atom’) (Not ‘to Al ion’) (Not ‘e–s transferred’)
[8]
|
|
|
© helpiks.su При использовании или копировании материалов прямая ссылка на сайт обязательна.
|