
- Автоматизация
- Антропология
- Археология
- Архитектура
- Биология
- Ботаника
- Бухгалтерия
- Военная наука
- Генетика
- География
- Геология
- Демография
- Деревообработка
- Журналистика
- Зоология
- Изобретательство
- Информатика
- Искусство
- История
- Кинематография
- Компьютеризация
- Косметика
- Кулинария
- Культура
- Лексикология
- Лингвистика
- Литература
- Логика
- Маркетинг
- Математика
- Материаловедение
- Медицина
- Менеджмент
- Металлургия
- Метрология
- Механика
- Музыка
- Науковедение
- Образование
- Охрана Труда
- Педагогика
- Полиграфия
- Политология
- Право
- Предпринимательство
- Приборостроение
- Программирование
- Производство
- Промышленность
- Психология
- Радиосвязь
- Религия
- Риторика
- Социология
- Спорт
- Стандартизация
- Статистика
- Строительство
- Технологии
- Торговля
- Транспорт
- Фармакология
- Физика
- Физиология
- Философия
- Финансы
- Химия
- Хозяйство
- Черчение
- Экология
- Экономика
- Электроника
- Электротехника
- Энергетика
Ethanol production
Ethanol production is among the oldest technology and is produced commercially by fermentation of cereal grains, molasses or other materials with high starch and/or sugar contents. The fermentation process involves conversion of sugars to alcohol and carbon dioxide by microorganisms.
The principal biological agents of fermentation are yeasts belonging to the genus Saccharomyces, which can catalyze alcoholic fermentation to ethanol production. In recent years, yeast strains of Saccharomyces cerevisiae were extensively studied for biotechnological properties enhanced.
Main sources for ethanol production
There are two main sources for obtaining ethanol.
Synthesis (catalytic hydration in vapor phase) and fermentation. The major part of production of ethanol is by fermentation (96%). The formed products are ethanol and carbon dioxide from sugar (glucose) and enzymes.
C6H12O6 + enzymes = 2 CH3CH2OH + 2 CO2
The enzymes come from yeast. In the industry of ethanol various yeasts can be used including species of Saccharomyces, Kleyveromyces and Candida, but most ethanol is produced by Saccharomyces.
Feedstock used for ethanol production
Ethanol production occurs from various biological feedstocks, which contain appreciable amount of sugar. These feedstocks can be classified as (i) monosaccharides or simple sugars that are used directly for fermentation and (ii) polysaccharides or more complex sugars that need to be hydrolyzed before fermentation (Fig. 5) with increased ethanol production costs.
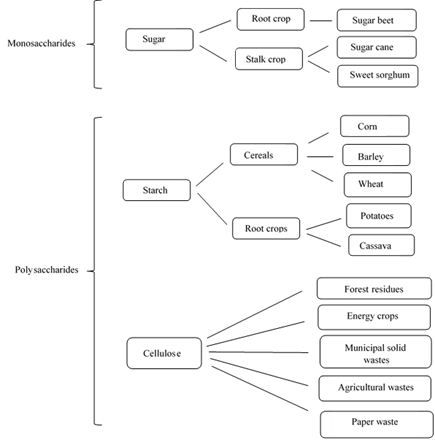
Figure 5. Feedstocks for ethanol production.
The various substrates used for ethanol production and their processing are briefly summarized below.
1. Sugar crops, e. g., sugarcane, sugar beet, sorghum, etc. provide a good substrate. Juices from these crops contain simple fermentable sugars, e. g., sugarcane juice has about 12% sugars.
2. Sugars from crop processing, e. g., molasses, sweet sorghum syrup, and spent sulphite liquor are the most common substrates. Molasses obtained after sugar recovery contains around 60% total fermentable sugars. Molasses is first suitably diluted before being used for fermentation.
3. Cereals as maize, wheat, sorghum, etc. contain 60-75% w/w starch. Generally, starch is a mixture of amylose (20-30%; water-soluble linear polymer) and amylopectin (70-80%; water-insoluble branched polymer).
4. Tubers like cassava, yams, potato etc. are rich in starch (30% on fresh weight basis). Cassava tubers are washed, mashed to pulp and subjected to liquefaction and saccharification in a manner similar to cereals.
5. Cellulosic substrates are the most abundant. The main components are cellulose and hemicellulose.
The great majority of ethanol produced in the world is from sugarcane molasses. The use of molasses for ethanol production has attracted great interest because they are low cost, rich in sucrose and all salts that microorganisms require for their growth. In addition, their substrate does not require pre-treatment prior to the fermentation.
Ethanol produced from parts of plant (materials that contain starch, sugar) is called ethanol of first generation feedstocks and ethanol of second generation provides utilization of whole plant (grains, tubes, stalks). Ethanol can be produced from biomass, which contain cellulosic and hemi cellulosic material, they are converted into sugar more difficult than conversion of starch into sugar. Cellulosic agriculture wastes include wheat straw, corn stover (leaves, stalks and cobs), rice straw and bagasse (sugar cane waste). Some cellulosic waste materials are grown especially for ethanol production such as poplar, willows and switch grass, percentage of cellulosic material in these ptudiedlants are between 30% and 70%.
Sugar cane as raw materials for ethanol production
Sugar cane (Saccharum officinarum) can be found in America, Europe, Asia, and Africa. The major producers are Brazil, India, China, Pakistan, Mé xico, Thailand, Columbia and Cuba.
Sugarcane has high sucrose gratified. The three main by products during the sugar production are bagasse, molasses and press mud. Molasses is a dark colored sugary remainder gotten from sugarcane after removal of all commercially profitable sugar. The molasses ingredients depends on the soil, climate, cane type and sugar refining process. Sugar concentration in molasses is about 50-66 wt %.
The production of ethanol from molasses is a well-established process.
Technological scheme for ethanol production from molasses
Typically, fermentation of 100 g glucose by selected strains of Saccharomyces cerevisiae yields 45-49 g ethanol, the theoretical limit being 51. 1 g (C6H12O6 = 2 CH3CH2OH + 2 CO2).
The Melle-Boinot process is the typical process for ethanol production by batch fermentation. In Fig. 6 is presented the technological scheme of ethanol production from molasses.
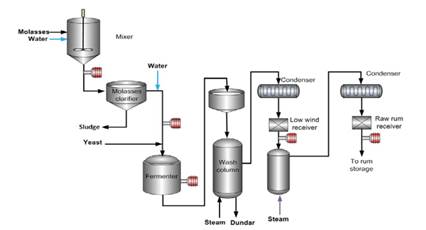
Fig. 6. Technological scheme for ethanol production from molasses.
Molasses should be diluted in adequate concentration to be assimilated by yeast. The pH is adjusted according to requirement of used microorganisms. Inoculum propagation is very important before starting fermentation. The aim is to obtain enough amount of cell biomass for inoculating the bioreactors. A yeast pure culture (Saccharomyces cerevisiae) is propagated. They are facultative anaerobic microorganisms (they can grow in aerobic and anaerobic conditions).
In the presence of oxygen, and at low sugar concentration, all the carbon sources are used for energy production and cell reproduction. There is an intensive biomass growth. Then is necessary full aeration, acidic pH, adequate sugar concentrations and nutrients. In addition, sterile conditions are necessary to ensure the presence only of desired microorganisms or to avoid contamination.
Fermentation is bio catalytic process, which occurs anaerobically. The sugar concentration (as total soluble solids) is adjusted to 150-200 g/L (15-200 Brix). In this step yeast convert sugars into ethanol, carbon dioxide and energy. Here sugar concentration is higher than during inoculum preparation. The necessary enzymes for degrading glucose to ethanol come from yeast Saccharomyces cerevisiae. These microorganisms grow in anaerobic conditions and acidic pH. In this step is not necessary sterile conditions, but nutrients are added.
When the fermentation is finished, the yeast cells are separated by centrifugation. They can be recycled in the fermentation process to increase the economical efficiency. The supernatant (wine, must) is pumped to the separation system. Ethanol is obtained by heating a fermented liquid at a high temperature that volatilizes most of the alcohol (distillation).
The distillation is based on the difference in boiling points of components of liquid. The boiling point of ethanol is 78 0C. Distillation columns are made up of a series of plates (Fig. 7). The feeding stream enters the column, and flows over the plates. As liquid goes down, vapor is exposed to it many times. Evaporation of ethanol occurs, and the ethanol-enriched vapor goes up to the top of the column. The alcohol is then condensed and collected. Final ethanol concentration: 96%
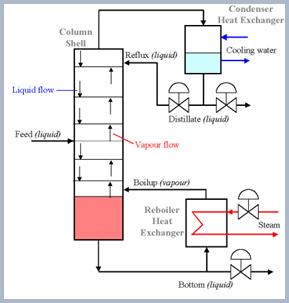
Fig. 7. Scheme of distillation stage.
By using multiple or repeated batch fermentation, the use of flocculating yeast strains plays an important role. In this process, after starting a conventional batch, the yeasts are decanted in the same vessel where they were cultivated by removing the clarified culture broth. Then, an equal amount of fresh culture medum is added for the following batch. In this way, high cell concentrations are reached and inhibition effect by ethanol is reduced without the need of adding flocculation aids or using separation or recirculation devices.
These repeated batches can be carried out until the moment when the metabolic activity and viability of culture is lost as a consequence of an inhibition of cells metabolism to the fermentation environment factors.
The batch process is the most commonly used and final yields are much higher than in other modes of operation.
Applications
Produced ethanol can be used in the industry as pharmaceutical component, chemical reagent, cosmetic, as food additives in the preparation of essences and flavorings (15%), as beverages (8%), as fuel and gasoline additive in the automotive transport (77%), among other uses.
The major part is used as fuel ethanol. Ethanol has a potential market as large as that of petroleum, since in addition to its energy uses it can also be used as a raw material for the production of substitutes for different petrochemical products.
The use of ethanol as fuel is increasing continuously. We will study the production of fuel ethanol from lignocellulosic materials, as part of biological waste treatments.
|
|
|
© helpiks.su При использовании или копировании материалов прямая ссылка на сайт обязательна.
|