
- Автоматизация
- Антропология
- Археология
- Архитектура
- Биология
- Ботаника
- Бухгалтерия
- Военная наука
- Генетика
- География
- Геология
- Демография
- Деревообработка
- Журналистика
- Зоология
- Изобретательство
- Информатика
- Искусство
- История
- Кинематография
- Компьютеризация
- Косметика
- Кулинария
- Культура
- Лексикология
- Лингвистика
- Литература
- Логика
- Маркетинг
- Математика
- Материаловедение
- Медицина
- Менеджмент
- Металлургия
- Метрология
- Механика
- Музыка
- Науковедение
- Образование
- Охрана Труда
- Педагогика
- Полиграфия
- Политология
- Право
- Предпринимательство
- Приборостроение
- Программирование
- Производство
- Промышленность
- Психология
- Радиосвязь
- Религия
- Риторика
- Социология
- Спорт
- Стандартизация
- Статистика
- Строительство
- Технологии
- Торговля
- Транспорт
- Фармакология
- Физика
- Физиология
- Философия
- Финансы
- Химия
- Хозяйство
- Черчение
- Экология
- Экономика
- Электроника
- Электротехника
- Энергетика
Actual Problems of Chemistry. Biotechnology. Lecture No. 2. Production of enzyme and ethanol. Enzyme technology
Actual Problems of Chemistry
Biotechnology
Lecture No. 2. Production of enzyme and ethanol
Last time we started to study some topics about Biotechnology. We were discussing some important aspects of bioprocess technology and the main steps of fermentation process.
In bioprocesses, a large numbers of cells are grown under defi ned conditions and it occurs inside the bioreactor. The correct medium composition and environmental growth-regulating parameters are factors that need to be controlled. In case of aerobic processes, it is important to control a good mixing and aeration.
We also, began to study the enzyme technology and were talking about the general characteristics of the enzymes together with the most important factors, affecting their catalytic activity.
In this lecture, we will study how to produce enzymes and ethanol. When we finish this activity, you should be able to:
Ø To describe the most common technology for the synthesis of both products
Ø To offer some applications
Enzyme technology
Most traditional biotechnological processes such as obtaining yogurt, beer production or grape fermentation to make wine are made by the enzymes that each microorganism produces for its particular metabolism. However, it is also possible to perform biotechnological processes with enzymes, in the absence of microorganisms.
Enzyme technology is primarily engaged in the production, isolation, purification and use of enzymes either in the soluble or immobilized form, for the benefit of humankind. With the advancements in, recombinant DNA technology, enzyme engineering produces more effective and diverse group of enzymes with useful applications in microbiology, biochemistry, diagnostics, therapeutics, biocatalysis, structural biology etc. The overall objective of this emerging technology is to produce unique sustainable products with specific function to fulfill the need of growing population.
Enzymes are obtained from microorganisms (bacteria, fungi or yeasts) selected by screening and harvested by fermentation procedures (reactors or flasks). Enzyme purification is achieved from the fermentation broth to yield the biocatalyst of commercial interest. The purification procedure involves any chromatographic and concentration techniques ensuring minimal loss of enzyme activity.
All stages for enzyme production are usually grouped under the term " biocatalytic cycle" and it is shown in Fig. 1.
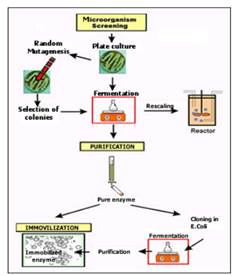
Fig. 1. Enzyme obtention process.
Selection of organism
It depends on the ability of microorganism to yield increased concentration of desired enzyme in a short time period with decreased amounts of secondary metabolites. Microbial inoculums are introduced in sterilized liquid medium for fermentation, providing optimal growth conditions such as pH, temperature, O2 supply, and nutrients.
Microbial enzymes are typically produced in batches by culturing the producing organism within a batch fermenter. Fermentation typically lasts between 30 and 150 h, with the optimum enzyme yield for the process falling somewhere between the optimum biomass yield and the point of maximal enzyme activity within the cells. Relatively small fermenters with a volume of 10–100 m3 are generally employed, allowing fl exibility where a number of diff erent products are being produced.
At the end of a fermentation in which a microorganism rich in the required enzyme has been cultured, the broth may be cooled rapidly to 5°C to prevent further microbial growth and stabilize the enzyme product. The pH may also be adjusted to optimize enzyme stability. If the enzyme-producing organism is a fungus, this may be removed by centrifugation at low speed. If the enzyme source is bacterial, the bacteria are often fl occulated with aluminum sulfate or calcium chloride, which negate the charge on the bacterial membranes, causing them to clump and thus come out of suspension.
Isolation and purification of enzyme
Desired enzyme produced may be excreted into the culture medium (extracellular enzymes) or may be present within the microbial cells (intracellular enzymes). It is recovered and purified by cell disruption techniques and downstream processing respectively ensuring minimal loss of enzyme activity.
Within the cell, enzymes are generally found along with other proteins, nucleic acids, polysaccharides and lipids. The activity of the enzyme in relation to the total protein present (i. e. the specific activity) can be determined and used as a measure of enzyme purity. A variety of methods can be used to remove contaminating material in order to purify the enzyme and increase its specific activity. Enzymes that are used as diagnostic reagents and in clinical therapeutics are normally prepared to a high degree of purity, because great emphasis is placed on the specificity of the reaction that is being catalyzed. Clearly the higher the level of purification, the greater the cost of enzyme production. In the case of many bulk industrial enzymes the degree of purification is less important, and such enzymes may often be sold as very crude preparations of culture broth containing the growth medium, organisms (whole or fragmented) and enzymes of interest.
A wide variety of techniques may be used for further purification. Chromatography is one of the most used practice.
Immobilization of enzyme.
During the production of commercially important products via enzymatic catalysis, soluble enzymes have traditionally been used in batch processes that employ some form of stirred-tank reactor. In these processes, at the end of the batch run the product must be separated from any unused substrate, and also from the enzyme catalyst. Removal of the enzyme at this stage can be achieved by thermal denaturation (only if the product is thermostable) or by ammonium sulfate precipitation or ultrafiltration. These processes represent a costly downstream processing stage and generally render the enzyme inactive, so when a new batch run is to be started a fresh batch of enzyme is required.
Immobilized enzyme systems, in contrast, “fix” the enzyme so that it can be reused many times, which has a significant impact on production costs.
The term “Immobilized enzymes” is defined as “Enzymes that is physically attached to specific solid supports and thus confined, and which can be used repeatedly and continuously while maintaining their catalytic activities”. In recent years, enzymatic productivity has been rapidly growing through the improvement of genetic engineering technology, microbial cultivation technology and wild type strain screening technology in parallel with the understanding of enzymatic biosynthesis mechanisms.
The use of immobilized enzyme in biotechnology has some advantages. First, a single batch of enzymes could be used multiply or repetitively. Second, immobilized enzymes are usually more stable than mobile enzymes. Third, the reaction could be controlled rapidly by removing the enzyme from the reaction solution. An additional advantage is the easy separation of the enzyme from the product so that contamination could be avoided.
In Fig2 is shown the methods to achieve enzyme immobilization.
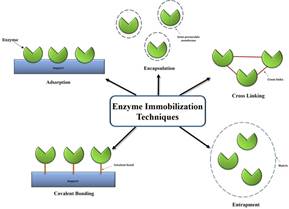
Figure 2. Various methods of enzyme immobilization.
• Adsorption: It involves the attachment of enzyme molecule on an inorganic or organic inert solid support such as silica gel, beads or glass, starch, cellulose etc.
The adsorption mechanisms are based on weak bonds such as Van der Waal's forces, electrostatic and hydrophobic interactions. Enzyme is dissolved in solution and the solid support is placed in contact with the enzyme solution for a fixed period of time under suitable conditions which sustain enzyme activity. The unadsorbed enzyme molecules are then removed from the surface by washing with buffer. Immobilization by adsorption is a simple and economical process which is reagent-free, low cost and is generally non-destructive toward enzyme activity because it does not involve any functionalization of the support. Nevertheless, this technique presents drawbacks: enzymes are loosely bound to the support by weak physical bonding so that changes in temperature, pH or ionic strength may result in enzyme desorption/leaching.
• Covalent binding: Enzyme immobilization by covalent binding is one of the most widely used methods, in which stable complexes between functional groups on enzyme molecules and a support matrix are formed through covalent bondings. The enzyme functional groups that could be utilized in covalent coupling include: Amino group, carboxylic group, phenolic group, sulfhydryl group, thiol group, imidazole group, indole group and hydroxyl group. The binding procedure of enzyme to the solid support generally goes through two stages: (1) activation of the surface using linker molecules such as glutaraldehyde or carbodiimide and (2) enzyme covalent coupling to the activated support. Linker molecules act as the bridge between surface and enzyme via covalent bonding. While the first group matches the immobilization surface and forms a so-called self-assembled monolayer (SAM), the second ground bound to preactivated support then forms a covalent bond with the enzyme. Covalent immobilization provides strong bindings between enzymes and support matrix and therefore little leakage of enzyme from the support may occur. In addition, high uniformity of the SAM layer and good control of the immobilized enzyme amount are the other advantages. In covalent attachment, there is a high risk of enzyme denaturation when most enzymes must go through chemical modifications to possess functional group. In addition, the method requires high volume of bioreagent but only small amounts of enzymes may be immobilized (~0. 02 grams per gram of matrix). The immobilization procedure largely increases enzyme stability but decreases enzyme activity in affinity reaction and is poorly reproducible. In comparison to adsorption, covalent bonding requires longer incubation time, since the formation of the SAM and the subsequent linkage of the enzymes to it take several hours. The process is also more complex and care has to be taken to ensure chemical purity so that the SAM is obtained in high homogeneity.
• Entrapment: enzymes can be immobilized by physical entrapment inside a mesh, capsule or a gel matrix of an inert material such as gelatin, polyacrylamide gel, starch, collagen, silicone, cellulose and rubber.
In entrapment immobilization, enzyme is not directly attached to the support surface but entrapped within a polymeric network which allows only the traverse of substrate and products but retains the enzyme hence enzyme diffusion is constrained. Entrapment immobilization process is conducted through two steps: (1) mixing enzyme into a monomer solution, followed by (2) polymerization of monomer solution by a chemical reaction or changing experimental conditions. As an enzyme is physically confined within a polymer lattice network, the enzyme does not chemically interact with the entrapping polymer. The method thus could improve enzyme stability and minimize enzyme leaching and denaturation. Another advantage of the method is the capability to optimize microenvironment for the enzyme by modifying the material to have the optimal pH, polarity or amphilicity. However, a limitation of the method is the mass transfer resistance occurred as polymerization extension tends to increase the gel matrix thickness, substrate for this reason can not diffuse deep into the gel matrix to reach the enzyme active site. Furthermore, the entrapped enzymes are likely to suffer from leakage if the pores size of the support matrix is too large. The method also has low enzyme loading capacity and the support material could be corrupted as effects of polymerization.
• Encapsulation method is performed by enclosing the enzymes in selectively permeable membranes, such as nitrocellulose or nylon. Encapsulation method is widely used for application in biomedical, food, detergents, wastewater treatment sectors. Generally, encapsulation is a simple and low cost immobilization method that can enhance the enzymatic activity, as there is large contact surface between enzyme and substrate.
• Cross-linking: is another irreversible method of enzyme immobilization that does not require a support to prevent enzyme loss into the substrate solution. The method is also called carrier-free immobilization where the enzyme acts as its own carrier and virtually pure enzyme is obtained eliminating the advantages and disadvantages associated with carriers.
Enzyme immobilization method is performed by the formation of intermolecular crosslinkages between the enzyme molecules by covalent bonds. The process is carried out with the assistance of a multifunctional reagent which acts as linkers to connect enzyme molecules into three dimensional cross linked aggregates.
Immobilization by crosslinking is a simple method which based on the strong chemical binding of enzyme biomolecules thus enzyme leakage is minimal. Another advantage of the method is the possibility to adjust microenvironment for enzyme by using suitable stabilizing agents through surface complementarity which helps increase stability.
In Fig. 3 is shown an example of different enzyme immobilization techniques. The crystal structure of Glucose Oxidase (GOx) isolated from Aspergillus Niger was used as a model enzyme. Physical and covalent immobilization techniques are discussed relative to flat nanosupport graphene and curved nanosupport, carbon nanotube respectively. Encapsulation is discussed relative to a pore geometry higher than the diameter of the enzyme, while cross-linking is illustrated relative to the distance between two individual GOx.
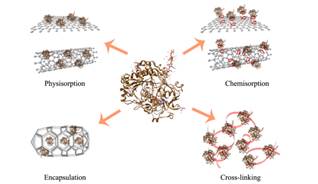
Figure 3. Schematic of different enzyme immobilization technique.
Once the method has been chosen, the optimization of the immobilization process is studied in order to achieve an industrial biocatalyst. Finally, best conditions for process are determined: optimal pH value and temperature, pH and temperature stability, determination of kinetic parameters and recycling.
Batch, Fed-batch and continuous cultivations are three major mode of operation for production of enzymes.
Scale-up issues is very important in commercial purpose from laboratory scale fermenters. Process optimization for the scale-up required the medium optimization to determine the eff ect of various defined ingredients as well as the complex nitrogen sources on enzyme production. Other fermentation conditions such as inoculum transfer, agitation, temperature for cultivation have also eff ect on optimized enzyme production for synthetic use.
Applications
Enzymes catalyze all kind of chemical reactions. Industrial and household catalysis becomes more and more dependent on enzymes. Industrial enzymes with the desired activity can be obtained by optimizing bioprocess technology conditions and by protein engineering. Enzyme engineering provides higher product quality, low manufacturing cost, low energy consumption and save time. This drives the market growth to enhance cost efficiencies and productivity, which grow the interest among consumers or customers.
According to Business Communication Company (BCC) Research, the global enzyme market is projected to grow from $5. 01 billion in 2016 to $6. 32 billion in 2021 (Figure 4), with market trends predicting a shift toward increased technical enzyme production including those used in textile, paper, leather, and biodiesel industries where excess waste generation incurs fines from environmental agencies. Such market projections were shown to be largely driven by process development in enzymatic biofuel production, which present good opportunities for the scale-up of immobilized biocatalysts.
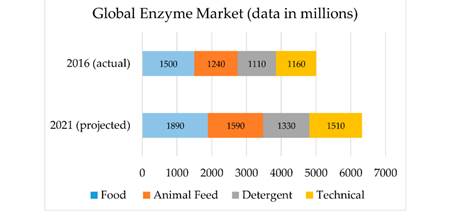
Figure 4. Global enzyme market in 2016 (top) and projected global enzyme market in 2021 (bottom).
Food and Beverage Industry
In many instances, traditional chemical synthesis routes are not viable for food products due to reagent toxicity and complex reaction chemistries that result in unfavorable process economics. Biocatalysts, on the other hand, present an opportunity for simplified, efficient production routes that mitigate the need for harsh substances, and thus are more economically competitive. As such, the use of biocatalysts in food and beverage processes dates back thousands of years to the advents of culinary practices like wine and cheese making. In modern times, the widespread use of enzymes in food and beverage industries for food quality preservation or modification is one of the earliest successful industrial applications of biocatalysis, observed in beer fermentation, juice debittering, and bread baking. The replacement of conventional chemical treatment with enzyme-catalyzed pathways for conversion of starch to glucose and fructose first took place several decades ago.
The conventional production route requires temperatures up to 175 0C and considerable pressurization, whereas biocatalytic processes can be carried out at temperatures near 100 0C and at ambient pressure via sequential α -amylase-catalyzed reactions encompassing both liquefaction and saccharification steps. In addition to milder reaction conditions, the multi-enzymatic process resulted in higher product selectivity and therefore allowed for better defined production routes for varying sugar products like maltose, fructose syrup, and crystalline sugar, as dictated by biocatalyst selection.
The production of fish protein hydrolysates from seafood processing waste via papain, a proteolytic enzyme derived from papaya that has found widespread industrial application, has garnered attention recently because the process is a potential solution for minimizing pollution from fishing industries.
Animal feeds
Enzymes used in animal feed must be able to attack the target substrate relatively quickly, as the passage through the animal’s digestive system is rapid, particularly for broiler chickens. The enzymes must also be able to work under the conditions of pH associated with the gastrointestinal tract, as well as being able to withstand the processing regime of the feed before it is fed to the animals. Most feed is pelleted, a process that involves conditioning of the feed with steam, followed by pressing through a die. The temperature at which this process occurs varies between 65 and 100 0C. Enzymes can be added directly to the feed as a dry product, in a premix containing vitamins and minerals, or applied after pelleting as a liquid if the regime of the feed mill is too harsh and may cause a loss of enzyme activity.
The most recent advances in feed enzymes have been aimed at improvements in the applicability and performance of phytases. These enzymes control phosphorus pollution and improve nutrient uptake. New fungal phytases have been identified with 4–50-fold higher specific activities than previously reported. Alternative approaches for the development of more effective enzymes have been to increase the catalytic activity of fungal phytases by site-directed mutagenesis; for example, on the basis of three-dimensional structural studies, the specific activity of the Aspergillus fumigatus phytase was increased fourfold. As is already known, in order for the enzymes to be applicable for use in pelleted feed products, they have to survive high temperatures during pelleting for short periods of time. A novel approach used to achieve thermal stabilization has been the construction of a ‘consensus phytase’ based on homologies among various phytases. This enzyme exhibits an increase in thermal stability to around 80°C.
Still, phosphorus utilization is not the only issue of concern to the animal feed industry; continuous effort is put into obtaining increased nutritional value from various feed sources, for example, by increasing the digestibility of the protein in soybean meal. It is likely that in the future we will see new and different hydrolytic enzymes applied in the feed industry to increase the value of feed stock, thus lowering the energy consumption and pollution per live stock to the benefit of the environment.
Detergent
Successful employment of biocatalysts is cited as the driving force of production of cost effective, environmentally benign detergents. In the instance of the detergents industry it should be noted that enzymes are a product rather than a chemical process-specific catalyst. Nonetheless, favorable market trends in the detergents’ industry reinforce the underlying view that biocatalytic products are inherently safer and more sustainable than traditional chemical products that pose health and safety risks.
Alkaline proteases, which are effective in the removal of protein stains and the cleaving of damaged cotton fibers, isolated from microbial sources comprise significant portions of multiple detergents produced and sold at commercial scale by manufacturers like Novozymes SA, Kao Corporation, and Genecor International. The high reaction specificity of enzymatic reactions further mitigates damage to fabrics and surfaces that is characteristic of chemically harsh detergent agents. Furthermore, the ratio of catalytically active enzymes in detergent mixtures are optimized for specific detergent applications; for instance, dishwashing detergents often contain varying degrees of amylase and lipase intended for the removal of starch food deposits and fats and oils, respectively. These enzymes quickly break down or release the dirt that normally can only be removed at much higher temperatures, or by using larger quantities of chemical detergents over a longer time period. Household detergents need to remove a broad range of complex soil from different fiber surfaces. Soil and stain components with good water solubility are easily removed during the cleaning process. Most other stains are partially removed by the surfactant/ builder/bleach system of a detergent, although the result is often unsatisfactory, depending on the washing conditions.
In most cases a suitable detergent enzyme aids the removal of soils and stains. Whereas the detergent components have a purely physicochemical action, enzymes act by degrading the dirt into smaller and more soluble fragments. However, to remove a stain totally still requires the joint action of the enzyme, the surfactant system, and mechanical agitation.
Technical industries
This segment comprises the starch, fuel alcohol, and other industries. The hydrolysis of starch and isomerization of glucose cannot be performed chemically at reasonable cost, as each would result in lower yields, unwanted by-products, and the considerable production of waste acids. In the other hand, one of the main challenges of second-generation biofuel production is identifying enzymes produced by microorganisms for use in a cocktail of enzymes to catalyze biomass hydrolysis in which the enzymes act together to break down the carbohydrates in sugarcane trash and bagasse, for example, and convert them into simple sugars for fermentation. The ethanol production will be studied as an important area of biotechnology application.
|
|
|
© helpiks.su При использовании или копировании материалов прямая ссылка на сайт обязательна.
|