
- Автоматизация
- Антропология
- Археология
- Архитектура
- Биология
- Ботаника
- Бухгалтерия
- Военная наука
- Генетика
- География
- Геология
- Демография
- Деревообработка
- Журналистика
- Зоология
- Изобретательство
- Информатика
- Искусство
- История
- Кинематография
- Компьютеризация
- Косметика
- Кулинария
- Культура
- Лексикология
- Лингвистика
- Литература
- Логика
- Маркетинг
- Математика
- Материаловедение
- Медицина
- Менеджмент
- Металлургия
- Метрология
- Механика
- Музыка
- Науковедение
- Образование
- Охрана Труда
- Педагогика
- Полиграфия
- Политология
- Право
- Предпринимательство
- Приборостроение
- Программирование
- Производство
- Промышленность
- Психология
- Радиосвязь
- Религия
- Риторика
- Социология
- Спорт
- Стандартизация
- Статистика
- Строительство
- Технологии
- Торговля
- Транспорт
- Фармакология
- Физика
- Физиология
- Философия
- Финансы
- Химия
- Хозяйство
- Черчение
- Экология
- Экономика
- Электроника
- Электротехника
- Энергетика
Pebbles. 27.6 Mechanism 2
Pebbles
18. 4 Radius: r = 10 / 6 = 1. 667 cm
18. 5 Number of layers: N = 50 / (2 × 1. 667) = 15
18. 6 Number of spheres: n = 15 × 7 = 105
18. 7 The volume of 105 spheres: 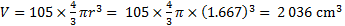
The fraction of volume: f = 2 036 / 3 927 = 0. 518, i. e. 51. 8 %
18. 8 The free volume: Vfree = 3 927 − 2 036 = 1 891 cm3
18. 9 The interlayer distance can be calculated as a height of a regular tetrahedron formed by 4 spheres with edge a = 2 × r (the formula can be simply derived from the Pythagorean theorem):
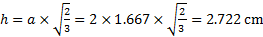
The distance of the first and the last layer from the bases of the cylinder will be at minimum equal to r. Thus the maximum number of layers:
N = (50 − 2 × r) / h + 1 = (50 − 2 × 1. 667) / 2. 722 + 1 = 18. 14 Þ 18 layers
18. 10 The total number of spheres: each of the 9 odd layers contains 7 spheres, each of the 9 even layers contains 3 spheres, the total number is:
n = 9 × 7 + 9 × 3 = 90
18. 11 The volume of 90 spheres: 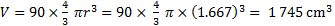
The fraction of volume: f = 1 745 / 3 927 = 0. 444, i. e. 44. 4 %
18. 12 The free volume: Vfree = 3 927 − 1 745 = 2 182 cm3
Sand
18. 13 The situation corresponds to the theoretical maximum possible space filling by spheres known as “close-packing of equal spheres”. The limiting fraction is 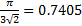 , i. e. 74. 05 %. There are many ways to derive this ratio. The derivation from a face centered cubic (fcc) elementary cell is shown.
, i. e. 74. 05 %. There are many ways to derive this ratio. The derivation from a face centered cubic (fcc) elementary cell is shown.
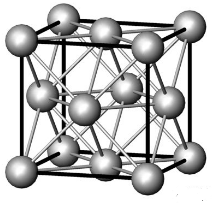
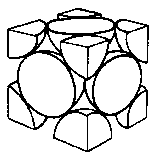
The lattice constant 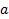 is
is  . Then the volume of the elementary cell is:
. Then the volume of the elementary cell is:
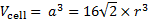
The number of spheres belonging to the elementary cell is:
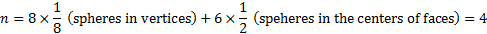
Thus the fraction volume occupied by the spheres is:
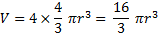
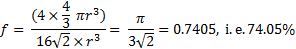
18. 14 The free volume: Vfree = 3 927 × (1 − 0. 7405) = 1 019 cm3
Problem 19. Structures in the solid state
19. 1 It is obvious from the picture that:
a(NaCl) = 2 × r(Na+) + 2 × r(Cl− ), and therefore:
r(Cl–) = ½ × (5. 64 − 2 × 1. 16) Å = 1. 66 Å.
19. 2 The density of KCl is:
r(KCl) = m / V = [4 × M(KCl)] / [NA × a(KCl)3], and therefore:
a(KCl) = {[4 × M(KCl)] / [NA × r(KCl)]}⅓ = [(4 × 74. 55) / (6. 022 × 1023 × 1. 98)]⅓ cm =
= 6. 30 × 10− 8 cm = 6. 30 Å
r(K+) = ½ × [a(KCl) − 2 × r(Cl− )] Å = ½ × (6. 30 − 2 × 1. 66) Å = 1. 49 Å.
19. 3 The ratio of ionic radii of Li+ to Cl− is:
r(Li+) / r(Cl− ) = 0. 90 / 1. 66 = 0. 54. It is higher than the relative size of the octahedral cavity (0. 41), which is a critical value for an ion to occupy this cavity. Thus, occupying this cavity by Li+ ion will result in a stable arrangement and LiCl should crystallize in the NaCl type of structure.
(Taking into account the smaller size of Li+ compared to Na+, it is not necessary to consider the upper limit of an ion size for a stable arrangement. However, for completeness, one can assume that the relative size of a cation with respect to an anion higher than 0. 73 should lead to the change of the coordination sphere and enforce a cubic coordination environment and the structure type of CsCl. In fact, the ratio for KCl is somewhat above this limiting value, but KCl still adopts the structural type of NaCl as stated above. )
19. 4 r (PbS) = m / V = [4 × M(PbS)] / [NA × a(PbS)3] = (4 × 239. 3) / (6. 022 × 1023 × 5. 943) g Å − 3 = = 7. 58 g cm− 3
19. 5 Due to total electro-neutrality, one can derive:
2(1 − x) + 1x = 2y, and thus:
y = 1 − ½ x
A general formula of silver-containing galena is thus Pb1–xAgxS1–½ x.
19. 6 r(Pb1− xAgxS1− ½ x) = m / V = [4 × M(Pb1− xAgxS1− ½ x)] / [NA × a(Pb1− xAgxS1− ½ x)3], and therefore:
M(Pb1− xAgxS1− ½ x) = r(Pb1− xAgxS1− ½ x) × NA × a(Pb1− xAgxS1− ½ x)3 / 4 =
= [7. 21 × 6. 022 × 1023 × (5. 88 × 10− 8)3] / 4 g mol− 1 = 220. 7 g mol− 1, and thus:
207. 2(1 − x) + 107. 9x + 32. 1(1 − ½ x) = 220. 7, and x = 0. 16
19. 7 Four.
19. 8 According to the crystal structure type, the bond distance of Ge–Ge corresponds to one fourth of the body diagonal of the unit cell and, thus, atomic radius is equal to one eighth of the body diagonal. Thus, the lattice parameter a(Ge) is:
a(Ge) = 8 × r(Ge) / √ 3 = 8 × 1. 23 / √ 3 Å = 5. 68 Å,
giving the density:
r(Ge) = m / V = [8 × M(Ge)] / [NA × a(Ge)3] = (8 × 72. 6) / (6. 022 × 1023 × 5. 683) g Å − 3 = 5. 26 g cm− 3
(Alternatively, substituting the a(Ge) by the term 8 × r(Ge) / √ 3 in the latter equation gives the following formula:
r(Ge) = m / V = [3√ 3 × M(Ge)] / [64 × NA × r(Ge)3] = 5. 26 g cm− 3
without the need to calculate the lattice parameter. However, the similarity of the lattice constant of Ge with that of the isoelectronic GaAs [a(GaAs) = 5. 65 Å ] is emphasized by the previous approach. )
19. 9 According to the crystal structure type, the bond distance of Ga–As (and Ga–P) corresponds to one fourth of the body diagonal of the unit cell. Thus:
d(Ga–As) = (5. 65 × √ 3) / 4 Å = 2. 45 Å
d(Ga–P) = (5. 45 × √ 3) / 4 Å = 2. 36 Å
The radius of phosphorus in these types of compounds is 0. 09 Å smaller than the radius of arsenic.
Problem 20. Cyclobutanes
20. 1 and 20. 2
Note that the two carbon atoms marked with a double asterisk (**) are pseudo-asymmetric. They have two constitutionally identical ligands which differ in configuration.
20. 3
20. 4
Problem 21. Fluorinated radiotracers
21. 1 18O (18-fluorine is synthesized by the following reaction: 18O + p → 18F + n)
21. 2 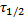 (18F) = 6 586 s
(18F) = 6 586 s
k = ln 2 / (6 586 s) = 1. 052 × 10− 4 s− 1
n = N / NA = 300 MBq / (1. 052 × 10− 4 s− 1 × 6. 022 × 1023 mol− 1) = 4. 73 × 10− 12 mol
21. 3 Heat of combustion of glucose = 2 800 kJ mol− 1
Chemical energy of one glucose molecule: Ec = 2 800 kJ / NA = 4. 650 × 10− 18 J
Energy of γ -photons per one glucose molecule: Ep = 2 × me × c2 = 1. 637 × 10− 13 J
Calculation of time:
Total chemical energy of 18O-glucose=Totalenergy of not yet released γ ‑ photons
Ec × N(glucose) = Ep × N(18F)
Ec × [N0(18F) − N(18F)] = Ep × N(18F)
Ec × N0(18F) × (1 − e− kt) = Ep × N0(18F) × e− kt
Ec = Ep × e− kt + Ec × e− kt
Ec / (Ec + Ep) = e− kt
ln [Ec / (Ec + Ep)] = − k × t
t = ln [(Ec + Ep) / Ec] × (1 / k) = ln (35 213) / (1. 052 × 10− 4 s− 1) = 9. 95 × 104 s = 27 h 38 min
21. 4 See structures below. X can be any K+ chelator (e. g. 18-crown-6 ether), not only [2. 2. 2]cryptand shown below.
21. 5
21. 6
21. 7
Problem 22. Where is lithium?
22. 1 The formation of organolithium reagents involves a radical pathway.
22. 2 The structures of intermediates A, B, C, and D:
22. 3 Reaction scheme for the haloform reaction:
Other reagents, such as NaOH + I2 or NaClO can also be used.
Problem 23. Synthesis of eremophilone
23. 1
23. 2 This reaction is called the Claisen rearrangement.
23. 3
Problem 24. Cinnamon all around
24. 1
24. 2 Direct UV irradiation (313 nm, acetonitrile). A conformationally mobile biradical is formed. Under these conditions, B and A are obtained in a 79: 21 ratio.
Alternatively, UV irradiation with sensitizers (e. g. riboflavin), or reagents such as diphenyldiselenide, hydrogen peroxide etc. can be used.
24. 3 Arbuzov reaction with 2-bromoacetic acid and tribenzyl phosphite:
24. 4
24. 5
24. 6 The carboxylic acid functional group reacts with DCC to form an O-acylisourea, which serves as the reactive intermediate in reactions with nucleophiles (e. g. alcohols or amines) in acyl nucleophilic substitutions.
24. 7 The starting compound is (E)-cinnamic acid methyl ester.
24. 8
24. 9 The two isomers Q and R are diastereoisomers (diastereomers).
24. 10 The acidic hydrogens of the OH groups would decompose the organolithium compound.
24. 11
24. 12 The reaction is named after Prof. Mitsunobu.
Problem 25. All roads lead to caprolactam
25. 1
25. 2 d)
25. 3
25. 4
25. 5 Gas E (NOCl) is orange. Therefore, the optimal wavelength would be below 530 nm (green and blue light).
25. 6 Beckmann rearrangement.
Problem 26. Ring opening polymerizations (ROP)
26. 1
26. 2
26. 3
26. 4 Ten grams of sodium ethoxide correspond to 10 / (2 × 12 + 5 × 1 + 1 × 23) = 0. 1923 mol. Two kilograms consumed with 83% conversion means 2 000 × 0. 83 = 1 660 g embedded into polymer. Each molecule of the initiator initiates one chain, so the number-average molecular weight is 1 660 / 0. 1923 = 8 632 g mol− 1. After rounding to two digits, we get the number-average molecular weight of 8 600 g mol− 1.
26. 5
26. 6
26. 7
26. 8
26. 9 A single wrong enantiomer of an amino acid in the protein structure causes loss of activity. Glycine is not chiral, so there are 129 − 12 = 117 chiral amino acids in lysozyme. The overall yield is (1/2)117 × 100% = 6. 02 × 10− 34 %.
Theoretically, in the “world behind the mirror” the all-d-protein would be active against the all-chiral reversed proteoglycan. However, this does not meet the condition that only the enzyme digesting native peptidoglycan is considered functional.
26. 10 The amount of enzyme (120 mg = 0. 000 12 kg) obtained with 6. 02 × 10− 34 % yield (see the the answer in 26. 9) would require the production of 0. 00012 / ((1/2)117) = 1. 99 × 1031 kg of material. As the Earth weighs 5. 972 × 1024 kg, this corresponds to 1. 99 × 1031 / 5. 972 × 1024 =
= 3. 34 × 106 times the mass of the Earth.
Problem 27. Zoniporide
27. 1
27. 2 Ammonia (NH3), carbon dioxide (CO2) and hydrogen (H2)
27. 3 and 27. 4
27. 5
27. 6 Mechanism 2
From a) the KIE is > > 1 which indicates that the C2–H bond is being cleaved during the rate determining step (RDS). For Mechanism 1 the RDS would have to be E → 3, for Mechanism 2 the RDS would be the concerted 2 → 3 transformation.
From b) we know that electron withdrawing groups (EWGs) on the heterocyclic core speed up the reaction. This indicates that the RDS involves either buildup of negative charge on the quinoline ring (e. g. by a nucleophilic attack) or loss of positive charge from the ring (e. g. by deprotonation). In Mechanism 1, this is true for the 2 → E step (an electron rich nucleophile adds to the quinoline core) but not for the E → 3 step (expulsion of a hydride nucleophuge is disfavoured in the presence of EWGs). This contradiction disproves Mechanism 1; therefore, the correct answer is Mechanism 2.
27. 7 Hydrogen peroxide (H2O2)
27. 8
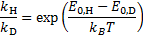
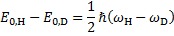
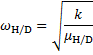
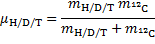
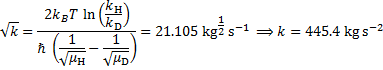
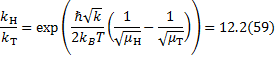
27. 9
Problem 28. Nucleic acids
28. 1
28. 2
28. 3
Unknown sample ( 1 ) transmittance: T1 = 0. 11
Known sample ( 2 ) transmittance: T2 = 1 − 0. 57 = 0. 43
Using Lambert–Beer law: − log10 T = ε l c
(− log10 T2) / c2 = (− log10 T1) / c1
c1 = c2 × (− log10 T1) / (− log10 T2) = 27 [μ mol dm− 3] (− log10 0. 11) / (− log10 0. 43) = 106 μ mol dm− 3
28. 4
a) True. According to the Lambert–Beer law, absorbance is directly proportional to concentration (as long as the cuvette length and the molar absorption coefficients are assumed equal). The higher absorbance of DNA1 actually means that the concentration of dsDNA1, which absorbs less radiation than ssDNA1, is lower.
b) False. Thermodynamic stability is described in terms of Tm, which can be read as the inflexion point of the sigmoidal curve; here Tm(DNA1) ~ 315 K and Tm(DNA2) ~ 340 K.
c) False. Since Tm(DNA1) ~ 315 K and Tm(DNA2) ~ 340 K, dsDNA2 is more stable than dsDNA1 with respect to their single-stranded forms.
d) Cannot be answered. The thermodynamic stability of a DNA double helix depends on both its length (i. e. the number of nucleobase pairs) and its sequence (roughly, the content of G–C nucleobase pairs). Since no information about the G–C pairs content is given, no conclusions about the DNA lengths can be drawn.
28. 5 cDNA: 5′ -ACCTGGGG-3′, mRNA: 5′ -CCCCAGGU-3′
28. 6 Each position of the 8-nucleobase sequence can be occupied by one of the four nucleobases (A, C, G, U). Hence, there are 48 = 65 536 theoretically possible
single-stranded octanucleotides.
|
|
|
© helpiks.su При использовании или копировании материалов прямая ссылка на сайт обязательна.
|