
- Автоматизация
- Антропология
- Археология
- Архитектура
- Биология
- Ботаника
- Бухгалтерия
- Военная наука
- Генетика
- География
- Геология
- Демография
- Деревообработка
- Журналистика
- Зоология
- Изобретательство
- Информатика
- Искусство
- История
- Кинематография
- Компьютеризация
- Косметика
- Кулинария
- Культура
- Лексикология
- Лингвистика
- Литература
- Логика
- Маркетинг
- Математика
- Материаловедение
- Медицина
- Менеджмент
- Металлургия
- Метрология
- Механика
- Музыка
- Науковедение
- Образование
- Охрана Труда
- Педагогика
- Полиграфия
- Политология
- Право
- Предпринимательство
- Приборостроение
- Программирование
- Производство
- Промышленность
- Психология
- Радиосвязь
- Религия
- Риторика
- Социология
- Спорт
- Стандартизация
- Статистика
- Строительство
- Технологии
- Торговля
- Транспорт
- Фармакология
- Физика
- Физиология
- Философия
- Финансы
- Химия
- Хозяйство
- Черчение
- Экология
- Экономика
- Электроника
- Электротехника
- Энергетика
The theoretical part
2. The theoretical part
The programmatic complex "FactSage" (version 6.4) was drawn on for the thermodynamics design of high temperature renewal of substance slag dumps [11]. The process was designed, where repairer was the hard carbon. Data about composition of slag, used in the process of design as initial, shown in the table 1. The array of the used data about composition of slags was got from literary sources and became the result of generalization of own analyses of composition slag dumps of Zlatoust metallurgical plant (Chelyabinsk area, Russian Federation). Some data about chemical and phase composition of these slags are driven to [8].
The FeO’s content makes the 15 masses slag (base composition is the second column of table 1). The magnetic separation is used for processing of slags, lowering maintenance gland in the slag, certain interest presents that, how lowering of maintenance of iron will affect on the result of restoration processes. The design was conducted for two compositions of slag with the lowered maintenance of FeO (third and fourth columns).
The simulations were performed for the temperature range 750-1650 ° C in step of 5 ° C at the pressure of the gas phase of 1 atmosphere. Thermodynamic calculation was performed on 100 g of slag. Simulations assumed that the system introduced deliberately excessive amounts of carbon (graphite). According to preliminary calculations, 100 g of slag elected enough to take 10 g of carbon.
Table 1 - Compositions of slags, used in the process of simulation
| Component of slag | Base compo-sition mass.% | Slag impoverished on Fe. mass, % | Slag strongly impo-verishhed on Fe. mass, % | Known results of experimental study. Mass, % |
| FeO | 15.00 | 10.00 | 5.00 | 3.72–43.88 |
| SiO2 | 25.50 | 27.00 | 28.5 | 17.7–26.6 |
| CaO | 30.00 | 31.76 | 33.53 | 21.9–47.4 |
| MgO | 11.40 | 12.07 | 12.74 | 6.2–18.5 |
| Al2O3 | 8.00 | 8.47 | 8.94 | 4.1–9.8 |
| MnO | 3.20 | 3.39 | 3.58 | 2.14–5.00 |
| Cr2O3 | 3.50 | 3.71 | 3.91 | 1.6–11.3 |
| TiO2 | 1.00 | 1.06 | 1.12 | 0.23–2.75 |
| V2O5 | 0.16 | 0.17 | 0.18 | 0.12–0.36 |
| NiO | 0.15 | 0.16 | 0.17 | 0.05–0.34 |
| Cu2O | 0.03 | 0.03 | 0.03 | less 0.05 |
| CaSO3 | 1.06 | 1.12 | 1.18 | 0.07–0.35 (content of sulfur) |
| Ca3P2O8 | 1.00 | 1.06 | 1.12 | 0.04–0.41 (content of phosphorus) |
| Sum |
Bases of FSstel, FToxid, FactPS is used for the design. The choice of solutions from their general amount came true in the process of preliminary design by the method of exception from the list of phases of variable composition those that does not prove as existing. Existence in the system of all substances from the used bases instead of duplicated (priority order - FSstel, FToxid, FactPS) was assumed being components select solutions (this exception is produced in the automatic mode) during the calculation. CaTiO3 is excluded from the list of clean substances (this substance is basis of solution of perofskite and not excluded from the list automatically).
The basic results of the conducted calculations are presented as dependences of the masses of components and compositions of the studied phases on the temperature for comfort of analysis.
Consider the simulation results of the renewal of basic slag composition.
Dependences of the masses of the basic condensed phases on the temperature are presented on the picture 1. The small amount (less 2 g.) of CaTiO3, sulfide of manganese, phosphate of calcium and FeV2O4 can be in the system.
Metallic fusion can appear already at temperatures 1070-1080 °С ensues from the picture 1. His amount with the increase of temperature grows (some falling down at temperatures about 1200 °C, but after again increasing) gradually. The liquid slag arises up in the system at the temperature about 1270 °C. His amount quickly grows and arrives at the maximum at the temperature about 1460 °C. The last hard oxide phase disappears at the same temperature in the system. Obviously, that to this temperature plenty of hard oxide phases will prevent to formation of the consolidated metallic fusion (to the association of drops of liquid metal), in spite of the fact that the fars of metallic fusion appear in the system at substantially less temperatures.
Substantial interest presents composition of metallic fusion appearing in the system. To estimate changes what be going on in this composition, allows the picture 2. The table of contents of iron in fusion maximally arrives at the size the about 90 mass %. according to the presented data in the period of origin of metallic fusion. The table of contents of iron falls with the increase of temperature, as other elements are restored and fill up by itself metallic fusion (it is chrome and manganese). The stake of cut-in in the metal carbon increases with the height of temperature.
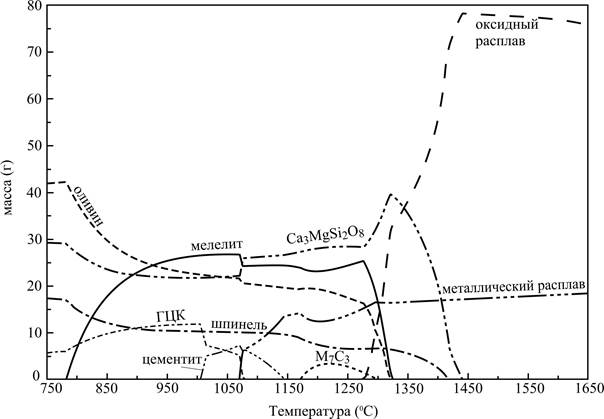
Figure 1 - Mass of condensed phases depending on temperature
Two groups can be distinguished by the nature changes of maintenance of minor admixture elements in composition a metal.
1) Nickel, phosphorus, copper and vanadium, maintenance of that (as well as maintenance of chrome) to the temperature 1350 °С goes out on the practically permanent level.
2) Elements maintenance of that in composition the metal originally extraordinarily small, however with the height of temperature increases steadily. Silicon, titan, aluminum and magnesium behave to the last group. The content of silicon grow and by 1650°С go out on the level of the 2-3 masses, %.
Iron is the basic component extractive in the process of renewal in the metallic phase. Calculations show that if at subzero temperatures iron is contained in composition different oxide phases (and also in composition the austenite), after, in an interval 1020-1340 °C a noticeable amount of iron is in the system as carbidic solutions, after 1340 °C practically all mass of iron is in composition metallic fusion. Iron is restored and goes across in the complement of fusion practically fully at such temperatures. Nickel and copper behave also. 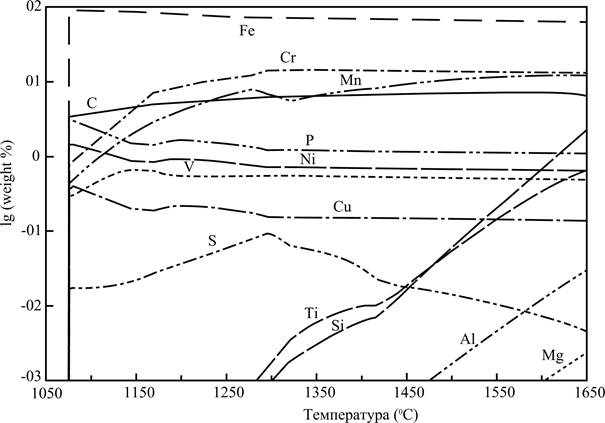
Figure 2 - composition of the liquid metal (mass fraction of components) depending on the temperature.
The manganese behaves other gates. The manganese is in the system as the solution of monooxides, solution of sulfide, in composition metallic fusion, and also as carbidic solutions to the temperature 1270 °C. Substantial part of manganese gets in slag fusion after 1270 °C, gradually restored and goes across in the complement of liquid metal with the height of temperature. The manganese is present in composition the slag in noticeable amounts to the high bound of the examined interval of temperatures. The content of manganese become noticeable and increases in the gas phase from temperatures about 1400 C. Design results show that on complete extraction of manganese in the complement of metallic fusion during renewal, expecting the carbon is not necessary. The noticeable amounts of manganese remain in composition the slag and gas even at maximal extraction of manganese in the metal.
Slag fusion presents most interest from all condensed oxide phases. It composition builds and presented, by oxides and sulfide components. Composition of slag changes with the height of temperature (due to renewal of heavy metals), but at the temperature an about 1470 °C maintenance of basic components (CaO, SiO2, MgO, Al2O3) go out on the permanent level - higher 41% CaO, about 33% SiO2, about 15% MgO and 9% Al2O3.
Composition of gas phase presents substantial interest. The design envisaged possibility of existence in composition the gas phase 95 substances. Gas composition is substantial from the point of view of ecological safety of process, taking into account the relatively large amount of appearing gas (about 10g. on 110g. of general mass of the system at the temperature 1500 °С)
Design of results are presented on the picture 3. Composition of gas phase is presented as denary logarithms of molefractions of components depending on the temperature.
Results designs specify on that in composition the gas phase carbon monoxide will prevail in all examined interval of temperatures. At temperatures necessary for formation of the consolidated metallic fusion (1450-1500 °С), maintenance another, except CO, components of gas phase less 1%. Large majority of components of gas phase is in it composition in quite negligible quantities.
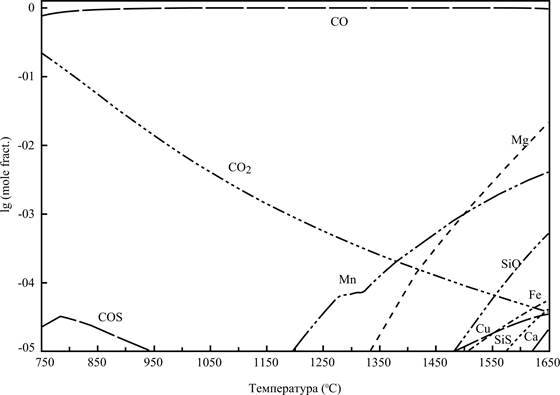
Figure 3 – Composition (decimal logarithm of the molar fractions) of gas phase
Pictures 4 and 5 allow to estimate as will affect on the basic parameters of process reduction of maintenance in the slag of iron.
The data presented on the picture 4 allow to conclude that treatment of slag with the lowered maintenance of iron will allow to get less of metallic fusion, but here will not affect fundamentally on his effectiveness.
Data of picture 5 allow to estimate the volumes of gaseous substances appearing in the process of renewal at different temperatures.
Information about the change of enthalpy of the system depending on a temperature allows to estimate the expenses of thermal energy, necessary for bring the system in the state at that interesting us processes become possible, presented also on this picture. The enthalpy of mixtures are equal DH0298(15%FeO) = –1218 kJ, DH0298(10%FeO) = –1266,3 kJ, DH0298(5%FeO) = –1315,7 kJ for the temperature 25 ºC.
300 kJ of thermal energy will be required for the transition from the state 110 grams of initial mixture on the basis of base composition at 25 ºC to the state of the system at 1500 ºC. The conducted calculation does not take into account thermal losses that the high temperature process will accompany, however and data can be useful, got for ideal terms.
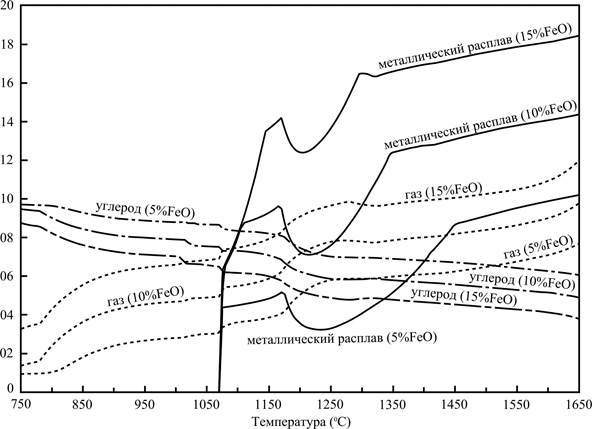
Figure 4 - Effect of slag composition on the masses of some phases
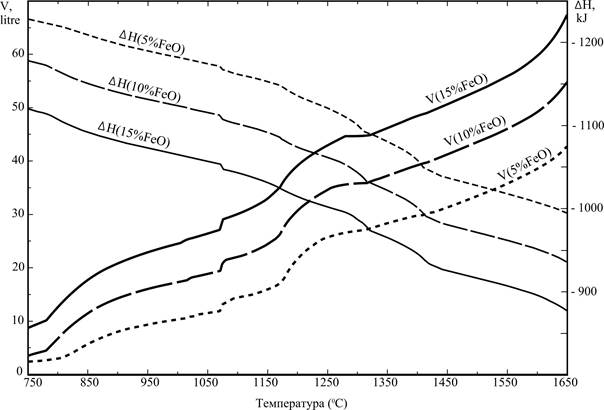
Figure 5 - Volume of the gas phase and enthalpy of the reaction mass, depending on the composition of the slag and temperature
3. Experimental part
The results of liquid-phase renewal are studied the carbon of slag materials in the process of experimental research, prepared on four different charts. The ground (in the ball mill to faction no more than 3 mm) up standard of dump steel-smelting slag was divided into magnetic and unmagnetic to faction, purged from the kinglets of metal, during the magnetic separation (by means of magnetic separator of drum type). Composition of factions is presented in the table 2, certain during the chemical analysis.
Table 2 - Chemical composition of slag fractions obtained during magnetic separation
| fractions | SiO2 | CaO | P | S | MgO | Al2O3 | MnO | Cr2O3 | TiO2 | V2O5 | FeO |
| magnetic | 25,6 | 31,6 | 0,2 | 0,34 | 11,9 | 10,6 | 3,20 | 2,00 | 0,80 | 0,10 | 14,5 |
| unmagnetic | 26,2 | 33,2 | 0,2 | 0,21 | 11,9 | 10,00 | 3,30 | 2,10 | 0,84 | 0,10 | 9,80 |
The half of material of every faction was exposed to solid-phase renewal, and then liquid-phase. Other part of standards at once was exposed to liquid-phase renewal. All standards of slag interfused with the coke in the mass relation 10: 2 (100 slag on 20 g of coke). The got mixture was exposed to careful interfusion.
Solid-phase renewal was performed as follows.
Standards in graphite glasses were placed in the stove of resistance, preliminary heated to 1100°С. Mode of circulation of air provided the restoration atmosphere in the zone of being of standards during all period of their being in the stove. Standards were maintained in the stove during 20 minutes after renewal of temperature of stove (after loading of standards she some fell) to 1100°C. Standards were taken out from the stove and cooled down on air in covered by the pieces of graphite glasses upon termination of the set time.
Prototypes in graphite crucibless were heated in the induction laboratory stove of YPI- 60-2 to the temperature 1500 C with self-control of temperature approximately at this level during 20 minutes at liquid-phase renewal. The visual supervision of molten slag mass in the process of self-control in the stove allowed to look after the process of it "boiling" intensity of that notedly went down by the end of period of treatment. 20 experience melting was conducted, that allowed to get the representative series of standards.
Crucibless with standards were extracted from working space of stove and cooled down on air, whereupon reactionary mass was extracted from crucible and investigated upon termination of time of self-control.
Determination of composition of phases was the aim of research of the got masses appearing during crystallization of products renewal, and also determination of stake of metallic product that is got in the process of renewal.
Determination of compositions of phases making the got marshes was conducted by means of scanning electron microscopes of JEOL JSM - 7001 and JOEL JSM - 6460 LV provided with analytical console.
The numerous images of surface of standards in secondary and reflected electrons were got, the semiquantitative and quantitative element analysis of points of surface and map of distribution of chemical elements was executed built during such sort of researches. The examples of photomicrographs of surfaces microsections of standards the marshes got in the process of renewal magnetic and unmagnetic factions of slag after liquid-phase renewal at the temperature 1500 °C are presented on the pictures 6 and 7. Chemical composition of separate (numbered) areas of these standards is driven to the tables 3 and 4.

Picture 6 - Standard of magnetic faction of original slag, recovered at 1500 °C. Scanning electron microscopes of JEOL JSM - 7001. The numbers of zones correspond to the table 3
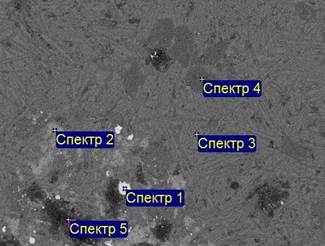
Picture 7 - Standard of unmagnetic faction of original slag, recovered at 1500 °C. Scanning electron microscopes of JEOL JSM, - 7001. The numbers of points correspond to the table 4.
Table 3 - Chemical composition (instead of carbon) of zones in the standard of magnetic faction of original slag, recovered at 1500 °C (the mass., % ).
| №, | O | Mg | Al | Si | Ca | Ti | V | Cr | Mn | Fe | Ni | Cu | Sum |
| 0,38 | 0,00 | 0,01 | 0,13 | 0,06 | 0,00 | 0,18 | 10,99 | 1,85 | 85,88 | 0,75 | 0,18 | 100,00 | |
| 35,87 | 10,39 | 2,32 | 16,62 | 21,99 | 0,68 | 0,12 | 1,95 | 8,99 | 1,29 | 0,00 | 0,00 | 100,00 | |
| 22,10 | 0,74 | 0,72 | 6,18 | 1,32 | 0,66 | 0,00 | 37,41 | 0,27 | 28,69 | 0,00 | 2,05 | 100,00 | |
| 11,81 | 0,10 | 0,11 | 0,25 | 0,37 | 0,00 | 0,00 | 0,67 | 1,30 | 85,75 | 0,13 | 0,12 | 100,00 |
Table 4 - Chemical composition (instead of carbon) of points in the standard of unmagnetic faction of original slag, recovered at 1500 °С (mass. %)
| №. | O | Mg | Al | Si | Ca | Ti | V | Cr | Mn | Fe | Ni | Cu | Sum |
| 2,72 | 1,21 | 0,28 | 0,70 | 0,17 | 0,00 | 0,10 | 3,39 | 0,43 | 91,12 | 0,08 | 0,12 | 100,00 | |
| 39,83 | 8,16 | 4,88 | 0,40 | 0,20 | 0,35 | 0,39 | 33,01 | 6,73 | 6,33 | 0,05 | 0,02 | 100,00 | |
| 47,79 | 15,37 | 5,68 | 17,29 | 7,67 | 0,34 | 0,01 | 1,12 | 3,58 | 0,39 | 0,06 | 0,00 | 100,00 | |
| 40,56 | 27,06 | 0,45 | 17,10 | 0,51 | 0,10 | 0,00 | 12,55 | 0,16 | 1,98 | 0,00 | 0,00 | 100,00 | |
| 21,03 | 3,31 | 0,75 | 1,67 | 1,33 | 0,03 | 0,06 | 61,98 | 3,49 | 5,12 | 0,26 | 0,84 | 100,00 |
The standards of marshes, got during experiments, were ground down and divided into the slag and metallic to part. Results of comparison of the masses metallic and slag parts (also gas, mass of that was calculated as difference of mass of standard to renewal and marsh got during this process) presented in the table 5. Some data of chemical analysis metallic and slag factions presented in the same table, allowing to judge about the depth renewals of metal.
Table 5 - Balance of distribution of the masses of standards phase-to-phase after liquid-phase renewal and some data about middle composition of the got phases.
| Type of the material exposed to renewal | The average content of Fe in the metal | The average content of Cr in the metal | The average content of Fe in the slag | The average mass fractions of the phases formed during renewal, % | ||
| metal | slag | gas | ||||
| Nonmagnetic without solid-phase renewal | 87.68 | 7.56 | 0,64 | 2.62 | 93.70 | 3.68 |
| Magnetic without solid-phase renewal | 89.39 | 5.22 | 1,64 | 8.16 | 85.91 | 5.93 |
| Nonmagnetic with solid-phase renewal at 1100 °С | 88.95 | 4.84 | 0,57 | 5.00 | 89.17 | 5.83 |
| Magnetic with solid-phase renewal at 1100 °С | 89.09 | 5.91 | 0,89 | 12.08 | 81.25 | 6.67 |
Metallic constituent in greater part of standards was presented as the small (1-4 mm by the diameter) kinglets of metal, plugged in slag mass it should be noted. The metal formed the consolidated metallic phase (bar) only at renewal of magnetic, preliminary recovered faction.
The results of electronic-microscopic standard of microsection of such bar are presented on the picture 8. The restored elements (Fe, Cr, Ni, Mn) are evenly up-diffused on all volume of standard.
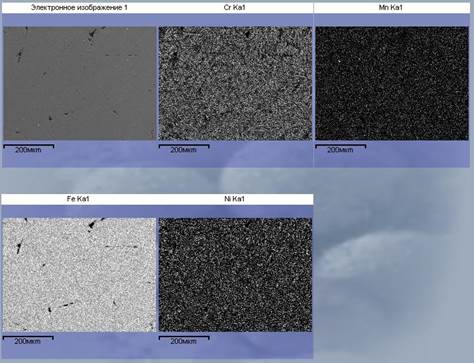
Figure 8 - Mapping of micro polished section of the metal’s bar, obtained during the renewal of the magnetic fraction of the slag. The increase x200
Etching of surface of their microsections was produced 4 solution of aquafortis in an alcohol for the exposure of structural features of the got standards of metal. Photos were got, allowing to judge about morphology of standard and about the processes of crystallization of metallic fusion at his cooling by means of microscope of C.Zeiss Observer D1m.
Photomicrograph of microsection of the metal, got after liquid-phase renewal testifies that this metal is characterized by the presence of plenty of pores and including of graphite, and slag measuring about 100 µm presented on the picture 9. The microstructure is educed, corresponding to the structure of hypoeutectic (ledeburite + pearlite) and hypereutectic (ledeburite + cementite) cast-iron after an etch on the surface of microsection of standard (picture 10). The simultaneous origin of hypoeutectic and hypereutectic cast-iron in the studied standards is related to the non-equilibrium process of crystallization of metallic fusion.

Figure 9 - Surface of microsection of the metallic including after etching
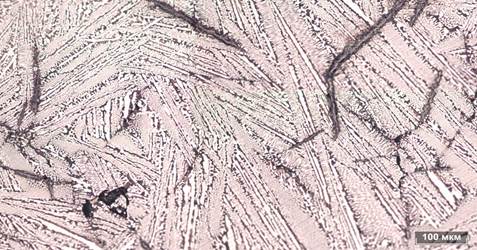
Figure 10а - Eutecticum cast-iron, graphite (dark plates) and nonmetallics. Increase x200

Figure 10b - Eutecticum cast-iron, graphite (dark plates) and nonmetallics. Increase x350
|
|
|
© helpiks.su При использовании или копировании материалов прямая ссылка на сайт обязательна.
|