
- Автоматизация
- Антропология
- Археология
- Архитектура
- Биология
- Ботаника
- Бухгалтерия
- Военная наука
- Генетика
- География
- Геология
- Демография
- Деревообработка
- Журналистика
- Зоология
- Изобретательство
- Информатика
- Искусство
- История
- Кинематография
- Компьютеризация
- Косметика
- Кулинария
- Культура
- Лексикология
- Лингвистика
- Литература
- Логика
- Маркетинг
- Математика
- Материаловедение
- Медицина
- Менеджмент
- Металлургия
- Метрология
- Механика
- Музыка
- Науковедение
- Образование
- Охрана Труда
- Педагогика
- Полиграфия
- Политология
- Право
- Предпринимательство
- Приборостроение
- Программирование
- Производство
- Промышленность
- Психология
- Радиосвязь
- Религия
- Риторика
- Социология
- Спорт
- Стандартизация
- Статистика
- Строительство
- Технологии
- Торговля
- Транспорт
- Фармакология
- Физика
- Физиология
- Философия
- Финансы
- Химия
- Хозяйство
- Черчение
- Экология
- Экономика
- Электроника
- Электротехника
- Энергетика
Name of sample. Atomic Ratio Pd/Sn. Binding Energy, eV
Type of the Paper (Article)
Preparation and Investigation of Pd and Bimetallic Pd‑ Sn Catalysts Supported on γ -Al2O3
IvanBondarchuk1, GrigoriyMamontov1, Irina Kurzina1 and FranciscoAires2, *
1 Department of Chemistry, National Research Tomsk State University; Russian Federation, 634050, Tomsk, 36 Lenin Ave.
2 Univ. Lyon, Université Claude Bernard Lyon 1, CNRS, IRCELYON – UMR 5256, 2, Avenue Albert Einstein, Villeurbanne F-69626, France.;
* Correspondence: francisco. aires@ircelyon. univ-lyon1. fr;
Received: date; Accepted: date; Published: date
Abstract: One of the key factors for producing highly active catalysts is the method used for metal deposition. The decomposition of metal-organic precursors is a good method for deposition of metal nanoparticles with a very small size and narrow size distribution on the surface of various supports. The preparation process of Pd and bimetallic Pd-Sn nanoparticles supported onto γ ‑ Al2O3 is considered. The samples were prepared by diffusional coimpregnation of the γ ‑ Al2O3 support by using organometallic Pd(acac)2 and Sn(acac)2Cl2 precursors dissolved in an organic solvent (toluene), followed by removal of the excess solvent. To achieve the formation of Pd and bimetallic Pd-Sn nanoparticles on the support surface, the synthesized samples were then subjected to thermal decomposition under Ar (to decompose the organomettalic bound to the surface while keeping the formed nanoparticles small) followed by an oxidation in O2 (to eliminate the organic compounds remaining on the surface) and a reduction in H2 (to reduce the nanoparticles oxidized during the previous step). A combination of methods was used to compare the physical-chemical properties of the synthesized Pd and bimetallic Pd-Sn nanoparticles supported on the γ -Al2O3 and showed that the goal of obtaining Pd and bimetallic Pd-Sn catalysts supported on γ Al2O3 was achieved.
Keywords: Bimetallic, Palladium-Tin catalysts, CO oxidation
1. Introduction
In recent years, the theoretical and experimental interest in bimetallic nanoparticles (BNPs) has risen sharply. BNPs are very attractive for use in various technological fields because of their unique physical-chemical properties. BNPs, in comparison with monometallic nanoparticles, have additional variable parameters, such as chemical and phase composition, structure and morphology, which allows better fine-tuning of magnetic, optical or catalytic properties for example[1-3]. BNPs are increasingly used in modern chemical technologies as highly effective catalysts. BNPs may exhibit increased catalytic activity, selectivity and stability as compared to their counterparts containing only one metal as suggested by recent studies [1-12; 4-5]. Bimetallic palladium-based nanoparticles can be used in a wide range of catalytic transformations to produce valuable organic compounds and fuels[6-7]. Therefore, considerable attention has been paid to the development of new methods for the synthesis of such nanoparticles in the past few years [13-188].
Incipient wetness impregnation – the most common used preparation method of supported catalysts has a significant disadvantage – the nonuniform deposition of the active component over the support granules. Particularly, this disadvantage is evident in the case of absence of adsorption interaction between the support and the precursor compound containing the active component. Obviously, preparation conditions such as solution viscosity, pH and the drying conditions of the catalyst have a significant influence on the distribution of the active component [19-209-10]. Diffusional impregnation by excess of the impregnation solution makes it possible to obtain supported catalysts which have a much uniform distribution over the grains of the support [2010]. The development of a preparation method for producing supported catalysts with a required distribution and optimum particle size of the active component is still relevant [2111].
The coordination and organometallic chemistry of palladium has developed very rapidly over past few decades. Palladium organometallic compounds are used as homogeneous catalysts to obtain a different intermediates in a wide range reactions of organic synthesis [2212]. Recently, organometallic palladium compounds have been utilized as high purity chemical compounds and precursors for the preparation of heterogeneous catalysts. Many authors make use of neutral palladium complexes that interact, generally from non-polar environment, with specific surface sites of the supports. Most often the acetylacetonate complex of palladium, Pd(C5H7O2)2 or simply Pd(acac)2, in inert solvents like benzene and toluene is used. Palladium acetylacetonatePd(acac)2 is a commercially available metal-organic complex used as a catalyst precursor [2313]. The preparation of Pd catalysts with 0. 5 wt% loadings by impregnation with bis-acetylacetonatePd(II) dissolved in toluene leads to the formation of metal particles having a very narrow size distribution centered near 4 nm [2414, 2515]. The particle size depends upon the activation treatment. The choice of the atmosphere and the sequence of oxidation-reduction treatments have a significant effect on the size of the supported nanoparticles. A direct decomposition under argon atmosphere leads to small particles with d ~ 5 nm. A decomposition of the impregnated Pd(acac)2 precursor under oxygen or hydrogen forms larger particles 15– 30 nm [2616]. Oxidation and subsequent reduction of such particles are necessary to obtain organic shell-free metallic supported particles suitable for catalysis.
Obtaining catalysts with a homogeneous composition is faced with the difficulty. As already been proposed in the case of the Pd-M system, the use of mixed salts as precursors of the bimetallic phase, in which both elements are associated in a molecular compound, can solve this difficulty. A facile pathway for preparation of BNPs is to synthesize mixed organometallic complexes directly on the surface support. After a calcination treatment to decompose the ligands and a reduction in hydrogen, bimetallic particles of 3 to 5 nm have been obtained. Indeed, previous works on Pd and PdCu supported on silica have shown that such an Ar treatment leads to metallic Pd and Pd-Cu bimetallic phases, well dispersed on the support. This cannot be easily achieved by classical co-impregnation methods [27-3217-22].
Among the many number of metal nanoparticles applied in heterogeneous catalytic reactions, bimetallic nanoparticles of palladium and tin are used successfully sufficient. Bimetallic Pd-Sn nanoparticles as heterogeneous catalysts are mainly used in selective hydrogenation of 1, 5-hexadiene and 1, 3-hexadiene [33-3523-25] 1, 3-butadiene [36-3826-28], citral [3929], 2-methyl-3-buten-2-ol [4030]. Bimetallic Pd‑ Sn nanoparticles supported on various supports for the denitration drinking water process have large perspectives [41-4631-36]. Also palladium-tin nanoparticles are potential catalysts in selective oxidation of CO in hydrogen [4737] and deep oxidation of methane [48-5238-42] and carbon monoxide [53-5643-46]. Currently, particular attention has been paid to the use of bimetallic Pd-Sn nanoparticles for the H2O2 synthesis [57-6147-51]. Consequently, catalytic systems based on Pd-Sn nanoparticles are of interest in various catalytic applications.
Thus, the aim of this study was to prepare supported Pd- and bimetallic Pd-Sn nanoparticles as well as to investigate their physical-chemical properties using ICP-OES, TEM, XPS and TPR-H2.
2. Results and Discussion
The results of the chemical analysis by ICP-OES are given in Table 2. The metallic content (Pdwt% or (Pd+Sn) wt%) is around 1. 3-1. 4wt% for the three catalysts. Comparing Tables 1 and 2 it appears that the measured metal content (Pd for Pd/γ ‑ Al2O3 and both Pd and Sn for PdSn/γ ‑ Al2O3 and Pd3Sn/γ ‑ Al2O3) is slightly higher than expected but within an acceptable margin. More importantly, for the two Pd-Sn samples the Pd/Sn atomic ratio is slightly lower than expected but within a less than 10% margin which is also acceptable. Thus this elemental analysis of the synthesized samples confirms the high efficiency of the loading of the active phase.
Table 1. Chemical composition and morphology analysis synthesized samples according to ICP-OES and TEM
| Title 1 | Title 2 | Title 3 |
| entry 1 | data | data |
| entry 2 | data | data 1 |
TEM observation of the three samples reveals the metal dispersion and allows to establish the particle size distributions. TEM micrographs illustrating the morphology of all samples supported on γ ‑ Al2O3 after the last preparation step (reductionat 500 °C during 2 h) are shown in Figure 1.
| (a) | (b) | (c) |
Figure 1. TEM micrographs of samples: ( a ) Pd/γ Al2O3; ( b ) Pd3Sn/γ Al2O3; ( c ) PdSn/γ Al2O3.
The bimetallic nanoparticles supported on γ ‑ Al2O3 forbothsamples PdSn/γ ‑ Al2O3 and Pd3Sn/γ ‑ Al2O3have a very narrow size distribution (2– 4 nm) withaverage particle size of 3 nm. In the case of Pd nanoparticles in Pd/γ ‑ Al2O3 the size distribution is broader (1– 6 nm). Actually, together with smaller particles (1– 3 nm) distribution, similar to the bimetallic samples, larger particles (4– 6 nm) also exist. It can be clearly seen that, from pure palladium to bimetallic samples, the average particle size decreases. The histograms of particle size distribution all the catalysts after reduction at 500 °C in hydrogen for 2 h are shown in Figure 2.
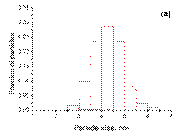 (a)
(a)
| 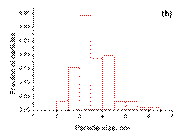 (b)
(b)
| 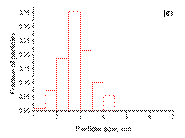 (c)
(c)
|
Figure 2. The histograms of particle size distribution: ( a ) Pd/γ ‑ Al2O3; ( b ) Pd3Sn/γ ‑ Al2O3; ( c ) PdSn/γ ‑ Al2O3.
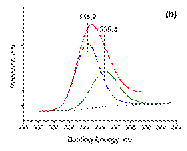
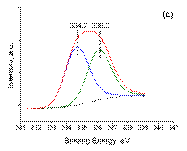
The surface composition and electronic properties of Pd and Sn on the γ -Al2O3 support were investigated by XPS. The XPS spectra for Pd3d of all the catalysts after reduction at 500 °C in hydrogen for 2 h are shown in Figure 3. For all samples, the binding energy of Pd3d5/2 is in the range of 334. 7– 335. 2 eV, indicating the presence of metallic palladium species [52-53]. For Pd/γ -Al2O3 and Pd3Sn/γ -Al2O3 (a sample where the nanoparticles are supposed to be Pd-rich) the binding energies of Pd3d5/2 were similar (335. 2 eV), indicating that both the phase of γ ‑ Al2O3and the size had no significant infl uence on the electronic properties of the metallic Pd-containing particles [28]. However, the binding energy of Pd3d5/2 for PdSn onγ ‑ Al2O3 is shifted towards a smaller value (334. 7 eV) indicating that Sn addition modifi es the electronic properties of Pd in this sample where nanoparticles are, contrary to Pd3Sn/γ -Al2O3 no longer Pd-rich [28]. A minor contribution of unreduced Pd (oxide) is also observed (BE~336. 3-336. 4 eV); this can be due to uncomplete reduction, as it is certainly the case for Pd/γ -Al2O3 and/or to the presence of tin for the bimetallic samples (this is adressed later in the text).
 (a)
(a)
|  (b)
(b)
|  (c)
(c)
|
Figure 3. The deconvoluted XPS spectra Pd3d5/2 core-level: ( a ) Pd/γ Al2O3; ( b ) Pd3Sn/γ Al2O3; ( c ) PdSn/γ Al2O3.
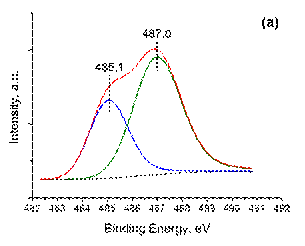
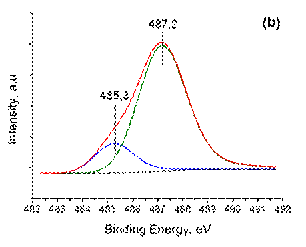
The deconvoluted XPS spectra of Sn 3d5/2 core-levelfor Pd3Sn/γ -Al2O3 and PdSn/γ -Al2O3are shown in Figure 4 and the (semi-)quantitative data extracted from these spectra are presented in Table 3. Both bimetallic samples demonstrate two similar components of tin species. The higher binding energy (487. 0– 487. 2 eV) can be related to tin oxide remaining even if the catalysts were reduced in hydrogen fl ow at 500 °C for 2 h. This is often the case due to (very) small unavoidable amounts of oxygen dead volume present in the vacuum locks during sample transfer and to the high propensity of Sn to oxidize. This observation together with the fact that part of tin is reduced simultaneously with palladium by TPR-H2suggests, as will be shown below, that the remaining unreduced tin is within the bimetallic nanoparticles. On the other hand, the remaining portion of tin component observed at the lower binding energy in the range 485. 1– 485. 3 eV can be assigned to the presence of palladium-tin alloy [43]. It can be seen also that with the addition of tin the amount of palladium (II) oxide increases for bimetallic Pd-Sn samples.
 (a)
(a)
|  (b)
(b)
|
Figure 4. The deconvoluted XPS spectra Sn3d5/2 (a) (b): ( a ) Pd3Sn/γ -Al2O3; ( b ) PdSn/γ -Al2O3 samples.
Surface compositionquantification and binding energy for all synthesized samples are given in Table 3. Surface analysis by XPS method showed after deposition one part of the supported Pd(acac)2 forms PdO species, the other part formed Pd(0) species. Formation of PdO can be attributed to strong interaction of metal precursor Pd(acac)2 with the γ -Al2O3 surface. Thermal decomposition under Ar inert environment and the successive oxidation-reduction treatments conduce to an increase Pd(0) amount due to the decomposition of acetylacetonate precursors and PdO reduction. The XPS results of the analysis shows that in bimetallic Pd-Sn samples there are two types of tin species, corresponding to Sn(0) species and to oxidized SnOx species. We cannot rule out the possibility of a composite Pd-Sn-O phase. It is necessary to notice that the addition of Sn induces an increase of PdO in the obtained Pd-Sn bimetallic nanoparticles. It may be assumed that the addition of Sn during the Pd-Sn bimetallic nanoparticles synthesis, supported onto γ -Al2O3, leads to a competitive reduction reaction of SnOx together with an inhibition of the PdO reduction.
Table 3. Surface analysis and binding energy for all synthesized samples by XPS
|
Name of sample |
Atomic Ratio Pd/Sn | Quantification, wt% |
Binding Energy, eV | ||||
| Pd | Sn | Pd3d5/2 (Pd) | Pd3d5/2 (PdO) | Sn3d5/2 (Sn) | Sn3d5/2 (SnOx) | ||
| Pd/γ ‑ Al2O3 | — | 0, 46 | — | 335, 2 | 336, 4 | — | — |
| Pd3Sn/γ ‑ Al2O3 | 3: 1 | 0, 48 | 0, 59 | 335, 2 | 336, 3 | 485, 1 | 487, 0 |
| PdSn/γ ‑ Al2O3 | 1: 1 | 0, 33 | 1, 67 | 334, 7 | 336, 2 | 485, 3 | 487, 2 |
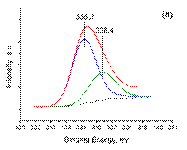
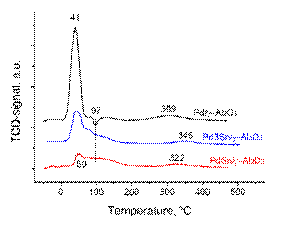
The TPR-H2 technique was used to investigate the reduction behavior of the samples. The obtained TPR profi les are shown in Figure 5. The all three samples exhibited TPR profi les with one main reduction peak which can be assigned to the reduction of PdO to Pd(0) metal [25]. The Pd/γ -Al2O3 sample was reduced at 41 °C while the reduction peak for both bimetallic Pd-Sn samples was slightly shifted to 50 °C. It is suggested that the smaller particles of Pd and both Pd-Sn samples, obtained from acetylacetonates precursors in toluene, resulted in a stronger interaction with γ ‑ Al2O3support. A large negative response at 97 °C for Pd sample can be attributed to the decomposition of Pd-β -hydride which is consistent with literature data [25]. This negative peak of Pd β -hydride decomposition indicates that some Pd particles formed a β -hydride during the initial steps – at low T) of the reduction.
It is interesting to note that Pd sample displayed extended small reduction peaks at the temperature range 200-400 °C, which may be attributed to reduction strongly bound palladium nanoparticles [54]. The TPR profiles of the bimetallic samples, shown below in Figure 5, show very different features compared to the monometallic Pd sample. In direct contrast, the both bimetallic samples did not showPd-β -hydride decomposition peakwhich implies that the presence of Sn is limiting the formation of this hydrogen containing Pd phase. Instead a positive peak was seen at 60-200 °C, as was observed for the Pd-Sn supported samples, but with no palladium-β -hydride decomposition peak. This reduction feature was split into a doublet which might indicate either the presence of two mixed phases or two closely separated reduction steps of a single species [55].
By contrast, in the case of both bimetallic Pd-Sn samples the intensity of TCD-signal decreased considerably with increasing Sn content (or. decreasing Pd content). Notably, the TPR profiles of the bimetallic Pd-Sn samples show a feature at 60-200°C. This feature is not present in the TPR profile of the Pd sample and is certainly related to an addition of the Sn species. When Sn is added to Pd, the TCD-signal between 60– 200 °C dramatically reduces and is accompanied by emergence of the positive TPR feature below 200 °C. This may represent the reduction of Pd-Sn bimetallic species [56].
The presence of this feature also coincides with the decrease in the intensity of the palladium-β -hydride signal, which may suggest that the some of the Pd species are no longer readily available to form palladium-β -hydride. These observations suggest that this unique TPR feature at 60-200 °C only present in the Pd-Sn bimetallic samples is likely to be related to a mixed Pd-Sn species, presumably a Pd-Sn alloy phase. This could either suggest that the presence of Sn inhibits the formation of palladium-β -hydride or that the Pd is present in a different form, such as a Pd-Sn alloy, which prevents it from forming palladium-β -hydride [29].
The onset of tin reduction is seen at 200 °C with bulk reduction beginning at around 450 °C [[И Б 1] 56]. Interestingly the TPR profiles of the bimetallic catalysts show a new feature at ~100 °C. This feature may be related to a lowering of the reduction temperature for the Sn species which occurred at 450 °C. An obvious feature of the TPR containing both Sn and Pd is the lower area of the Sn reduction peaks above 300 °C. The features present at temperature range 300-400°C have been assigned to the reduction of small surface Sn species [55-56].
 (a)
(a)
|
Figure 5. TPR profi les of all synthesized samples: ( a ) Pd/γ -Al2O3; ( b ) Pd3Sn/γ -Al2O3; ( c ) PdSn/γ -Al2O3.
4. Materials and Methods
4. 1. Preparation of the γ ‑ Al2O3 support
We have chosen a γ Al2O3 powder composed by 2 5 mm diameter spheres of spheres. The alumina balls were crushed in order to get a very fine powder (5. 0 g for each one of the three catalysts). The grinded powder was dried in an evacuated oven at 120°C for 24h. It was then sieved and a 0. 25 0. 5 mm fraction was selected. The BET surface area of γ Al2O3 supports were determined by N2 physical adsorption using a Micromeritics «TriStar 3020» automated system. The measured surface area was 335 m2/g and pore volume was 0. 44 cm3/g.
4. 2. Synthesis of Pd and bimetallic Pd-Sn nanoparticles on the γ ‑ Al2O3 surface.
As starting palladium and tin precursors, acetylacetonate complexes Pd(acac)2, Sn(acac)2Cl2 were used. Before diffusional impregnation for deposition precursors of the active component, the grinded powder γ Al2O3 was dried in a vacuum oven at 80°C for 24 hours. The nanoparticles preparation was carried out by diffusional impregnation of the support: pore volume of the γ Al2O3 was prefilled with using an organic solvent (toluene). For this purpose, the required amount of Pd(acac)2, Sn(acac)2Cl2 was dissolved in a large excess of toluene with continuous stirring. To the resulting solution, 5. 0 g of support γ -Al2O3 powder was added in the presence of toluene excess for 24 h. The excess solvent was removed on a rotavapor at 80°C for 30-60 min. Further evaporation of the remaining toluene was carried out in a vacuum oven at 80°C for 24 h. Finally, thermal and oxidation-reduction treatments were performed and included the following steps: (i) decomposition of the organometallic under Ar at 500°C for 2h to minimize the size of the nanoparticles; (ii) calcination under O2 at 350°C for 2h to eliminate the organic residus due to the previous decomposition; it should be noted that after this treatment the existing nanoparticles are oxidized; (iii) final reduction step under H2 at 500°C for 2h is thus necessary to obtain supported (bi)metallic nanoparticles; this was, of course, done after the evacuation of O2 under Ar for 30 min at 350°C. All temperature ramps were 1°C/min. The precalculated content of metals in the samples are given in Table 4.
Table 4. Theprecalculated theoretical content of metals in all synthesized samples
| Name sample | Ratio Pd/Snat. % | m(Pd), mg. | m(Sn), mg. | ω (Pd), wt. % | ω (Sn), wt. % |
| Pd/γ ‑ Al2O3 | ― | 60, 0 | ― | 1, 20 | ― |
| Pd3Sn/γ ‑ Al2O3 | 3: 1 | 43, 7 | 16, 3 | 0, 87 | 0, 33 |
| PdSn/γ ‑ Al2O3 | 1: 1 | 28, 4 | 31, 7 | 0, 57 | 0, 63 |
4. 3. Chemical elemental analysis - ICP-OES
The composition of catalysts was determined using an inductively coupled plasma-optical emission spectroscopy (ICP-OES) «Activa» Jobin Yvon. Pd was dissolved in a mixture of H2SO4 + aqua regia at 250-300 °C before analysis. Sn-containing samples were dissolved in a mixtutre H2SO4 + HNO3 at 250-300 °C then 20% HCl at room temperature.
4. 4. Electron microscopical study and local elemental analysis – TEM‑ EDX
The particle size distribution and the local chemical composition of the nanoparticles were determined by transmission electron microscopy (TEM) and energy-dispersive X-ray spectrometry (EDX). We used a JEOL JEM 2010, operating at 200 kV, equipped with a LaB6 thermoionic electron gun, a high resolution pole piece (0. 196 nm point resolution) and Pentafet Link-Isis EDX spectrometer from Oxford Instruments. The samples were diluted in ethanol, ultrasonicated and dispersed on an electron microscopy Cu grid (3. 05 mm, 300 mesh) coated with a holey-carbon film prior to the observation. The general morphology of the samples together with particle size distributions of Pd an PdSn nanoparticles were obtained from conventional TEM observations [48, 4957-58]; local structure could be obtained by high resolution imaging (HRTEM) [50]. Furthermore, local chemical analysis of the nanoparticles was carried out with EDX in the TEM [49-5158-60]
4. 5. Surface analysis - XPS
XPS analysis was performed with a Kratos Axis Ultra DLD spectrometer, equipped with a hemispherical analyzer, and a state of the art delay line detector. A monochromated Al-Kα X-ray source with charge neutralization was used. In order to reduce the samples prior to analysis, they were treated under H2 fl ow at 500°C for 2 h in a reaction chamber coupled to the ultra-high vacuum XPS chamber. The samples could then be transferred for XPS analysis without air contact and thus without further possibility of contamination. The Al 2p line was taken as an internal standard at 73. 4 and 73. 6 eV and confirmed by checking the binding energy (BE) of the principal component of the peak-fitted C1s envelope which is arbitrarily assigned the value of 284. 6 eV; this component is characteristic of C-C and C-H bonds that are generally present in the samples as contaminant carbon [52, 5361-62]. The precision in BE measurements was ±0. 1 eV for all samples.
4. 6. Temperature-programmed reduction in H2 – TPR-H2
The reactivity of samples with respect to H2was studied by temperature-programmed reduction TPR-H2 method. TPR-H2 measurements was carried out on a Micromeritics Chemisorb 2750 automated chemisorption analyzer system attached with ChemiSoftTPx software. Before analysis, the catalysts were oxidized in O2 fl ow at 500 °C for 2 h with temperature ramp 10°C/min. The TPR-H2was carried out in a Micromeritics Chemisorb 2750 automated system. A catalyst sample of 100 mg, a temperature ramping from − 50°C to 300 °C at 10°C/min, and a carrier gas composed of 10% H2 in Arwere used. During reduction, a cold trap was placed before the detector to remove water produced. A thermal conductivity detector (TCD) was used to measure the amount of hydrogen consumption.
5. Conclusions
In conclusion, we have described a perspective route to the preparation of bimetallic Pd-Sn nanoparticles and characterized their morphology and electronic structure. Bimetallic Pd-Sn nanoparticles s upported on γ -Al2O3 were prepared by the diffusional coimpregnation method displayed in this work using Pd(acac)2 and Sn(acac)2Cl2 as metal precursors because of their higher dispersion of nanoparticles they yield. The prepared catalyst contained ~1, 3wt% of active phase, which indicates that the preparation method is very effective in terms of content of Pd and Sn in the bimetallic samples. The TEM-EDX studyalsoconfirmsthatco-impregnationisanefficientandsimplemethodtoproducebimetallicPd-Sn nanoparticles, supported onto γ -Al2O3. Morphology analysis of obtained samples by TEM method showed for both Pd and bimetallic Pd-Sn samples that the supported nanoparticles have a small size with rather narrow uniform size distribution over the support surface. Thermal decomposition of acetylacetonate precursors leads to the formation of bimetallic nanoparticles where the of palladium both Pd(0) species and oxidized PdO species was devised by XPS. Notably, addition of the second element, which is an electron density promoter, leads to a change of the electronic state of the obtained particles and also to the formation of bimetallic species (containing or not oxygen). The addition of Sn conduces to an increased part of PdO of bimetallic Pd-Sn nanoparticles. It may be assumed that addition of Sn during the preparation of supported bimetallic Pd-Sn nanoparticles leads to a competitive reduction reaction of SnOx and, possibly, inhibition of the PdO reduction. The TPR profiles of the catalysts show that a new feature is present in the bimetallic samples and the absence of a Pd-β -hydride decomposition feature indicates that there is a strong interaction between the Sn and Pd in these catalysts. TPR shows Pd and Sn are likely to be mixed to form bimetallic species as the addition of Sn hinders the formation of Pd-β -hydride. The Pd-Sn bimetallic catalysts showed additional high temperature reduction peaks above 300 °C which are related to Sn reduction.
Supplementary Materials: The following are available online at www. mdpi. com/xxx/s1, Figure S1: title, Table S1: title, Video S1: title.
Author Contributions: For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “ conceptualization, X. X. and Y. Y.; methodology, X. X.; software, X. X.; validation, X. X., Y. Y. and Z. Z.; formal analysis, X. X.; investigation, X. X.; resources, X. X.; data curation, X. X.; writing— original draft preparation, X. X.; writing— review and editing, X. X.; visualization, X. X.; supervision, X. X.; project administration, X. X.; funding acquisition, Y. Y. ”, please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding: Please add: “ This research received no external funding” or “ This research was funded by NAME OF FUNDER, grant number XXX” and “ The APC was funded by XXX”. Check carefully that the details given are accurate and use the standard spelling of funding agency names at https: //search. crossref. org/funding, any errors may affect your future funding.
Acknowledgments: In this section you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e. g., materials used for experiments).
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
References must be numbered in order of appearance in the text (including citations in tables and legends) and listed individually at the end of the manuscript. We recommend preparing the references with a bibliography software package, such as EndNote, ReferenceManager or Zotero to avoid typing mistakes and duplicated references. Include the digital object identifier (DOI) for all references where available.
Citations and References in Supplementary files are permitted provided that they also appear in the reference list here.
In the text, reference numbers should be placed in square brackets [ ], and placed before the punctuation; for example [1], [1– 3] or [1, 3]. For embedded citations in the text with pagination, use both parentheses and brackets to indicate the reference number and page numbers; for example [5] (p. 10), or [6] (pp. 101– 105).
Author 1, A. B.; Author 2, C. D. Title of the article. Abbreviated Journal Name Year, Volume, page range.
Author 1, A.; Author 2, B. Title of the chapter. In Book Title, 2nd ed.; Editor 1, A., Editor 2, B., Eds.; Publisher: Publisher Location, Country, 2007; Volume 3, pp. 154– 196.
Author 1, A.; Author 2, B. Book Title, 3rd ed.; Publisher: Publisher Location, Country, 2008; pp. 154– 196.
1. Gilroy, Kyle D.; Ruditskiy, Aleksey; Peng, H. -Ch.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414-10472.
2. Shan, Sh.; Luo, J.; Yang, L.; Zhong, Ch. -J. Nanoalloy catalysts: structural and catalytic properties. Catalysis Science & Technology 2014, 4, 3570-3588.
3. Xu, Y.; Chen, L.; Wang, X., Yao, W.; Zhang, Q. Recent advances in noble metal based composite nanocatalysts: colloidal synthesis, properties, and catalytic applications. Nanoscale 2015, 7, 10559-10583.
4. Jianga, H. -L.; Xu, Qiang. Recent progress in synergistic catalysis over heterometallic nanoparticles. Journal of Materials Chemistry 2011, 21, 13705-13725.
5. Liu, X.; Wang, D.; Li, Y. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today 2012, 7, 448-466.
6. Liao, F.; Lo, T. W. B.; Tsang, S. C. E. Recent Developments in Palladium‐ Based Bimetallic Catalysts. ChemCatChem 2015, 7, 1998-2014.
7. Ellert, O. G.; Tsodikov, M. V.; Nikolaev, S. A.; Novotortsev, V. M. Bimetallic nanoalloys in heterogeneous catalysis of industrially important reactions: synergistic effects and structural organization of active components. Russ ChemRev 2014, 83, 718– 732.
8. Adriana Zaleska-Medynska; MartynaMarchelek; Magdalena Diak; Ewelina GrabowskaNoble metal-based bimetallic nanoparticles: the effect of the structure on the optical, catalytic and photocatalytic properties. Advances in Colloid and Interface Science 2016, 229, 80-107.
9. Pakhomov, N. A.; Buyanov, R. A. Current Trends in the Improvement and Development of Catalyst Preparation Methods. Kinetics and Catalysis 2005, 46, 669– 683.
10. Pakhomov N. A. The scientific basis for the preparation of catalysts: an introduction to theory and practice; Publishing Company SB RAS: Novosibirsk, Russia, 2011; pp. 172– 196.
11. Munnik, P.; de Jongh, P. E.; de Jong, K. P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687-6718.
12. Temkin, O. N. Homogeneous Catalysis with Metal Complexes: Kinetic Aspects and Mechanisms; Wiley-Blackwell: Oxford, United Kingdom, 2008; 830 Pages.
13. Toebes, Marjolein L.; vanDillen, Jos A., de Jong, Krijn P. Synthesis of supported palladium catalysts. Journal of Molecular Catalysis A: Chemical 2001, 173(1– 2), 75-98
14. Mé thivier, C.; Massardier, J.; BertoliniJ. C. Pd/Si3N4 catalysts: preparation, characterization and catalytic activity for the methane oxidation. Applied Catalysis A: General 1999, 182(2), 337-344.
15. Garcia Cervantes, G.; Cadete Santos Aires, F. J.; BertoliniJ. C. Compared properties of Pd on thermo-conductor supports (SiC, Si3N4) and Pd on oxide supports (Al2O3, SiO2) for the 1, 3-butadiene hydrogenation reaction. Journal of Catalysis 2003, 214(1), 26-32.
16. Mé thivier, C.; Bé guin, B.; Brun M.; Massardier J.; Bertolini, J. C. Pd/SiC Catalysts: Characterization and Catalytic Activity for the Methane Total Oxidation. Journal of Catalysis 1998, 173 (2), 374-382.
17. A. J. Renouprez; K. Lebas; G. Bergeret; J. L. Rousset; Delichere, P. Supported Pd-Cu catalysts prepared from bimetallic organo-metallic complexes: relation between surface composition measured by Ion Scattering and reactivity. Studies in Surface Science and Catalysis 1996, 101, 1105-1114.
18. Renouprez, A.; Lebas, K.; Bergeret, G. A new method of direct synthesis of bimetallic phases: Silica supported Pd-Cu catalysts from mixed acetylacetonates. Journal of Molecular Catalysis A: Chemical 1997, 120, 217-225
19. F. J. Cadete Santos Aires; R. Darji; J. F. Trillat; A. Howie; A. Renouprez. Hollow metallic particles obtained by oxidation/reduction treatment of organometallic precursorsStudies in Surface Science and Catalysis 2000, 130, 1109-1114.
20. A. J. Renouprez; J. F. Trillat; B. Moraweck; J. Massardier; G. BergeretPd-Mn Silica Supported Catalysts: 1. Formation of the Bimetallic Particles. Journal of Catalysis 1998, 179, 390-399
21. J. F. Trillat; J. Massardier; B. Moraweck; H. Praliaud; A. J. RenouprezReduction of NO by CO on Manganese promoted Palladium Catalysts. Studies in Surface Science and Catalysis 1998, 116, 103-112.
22. A. J. Renouprez; A. Malhomme; J. Massardier; M. Cattenot; G. BergeretSulphur resistant palladium-platinum catalysts prepared from mixed acetylacetonates. Studies in Surface Science and Catalysis 2000, 130, 2579-2584.
23. Emerson Andrade Sales; Guy Bugli; Alain Ensuque; Má rio de Jesus Mendes; Francois Bozon-Verduraz. Palladium catalysts in the selective hydrogenation of hexa-1, 5-diene and hexa-1, 3-diene in the liquid phase. Effect of tin and silver addition Part 1. Preparation and characterization: from the precursor species to the final phases. Physical Chemistry Chemical Physics 1999, 1, 491-498.
24. Emerson Andrade Sales; Mariode Jesus Mendes; Franç oisBozon-Verduraz. Liquid-Phase Selective Hydrogenation of Hexa-1, 5-diene and Hexa-1, 3-diene on Palladium Catalysts. Effect of Tin and Silver Addition. Journal of Catalysis 2000, 195, 96-105.
25. Emerson AndradeSales; Jose Jove; Mariode Jesus Mendes; Franç oisBozon-Verduraz; Palladium, Palladium– Tin, and Palladium– Silver Catalysts in the Selective Hydrogenation of Hexadienes: TPR, Mцssbauer, and Infrared Studies of Adsorbed CO. Journal of Catalysis 2000, 195, 88-95.
26. S. Verdier; B. Didillon; S. Morin; J. C. Jumas; J. Olivier-Fourcade; D. Uzio. Pd– Sn/Al2O3 catalysts from colloidal oxide synthesis: I. Preparation of the catalysts. Journal of Catalysis 2003, 218, 280-287.
27. S. Verdier; B. Didillon; S. Morin; D. UzioPd– Sn/Al2O3 catalysts from colloidal oxide synthesis: II. Surface characterization and catalytic properties for buta-1, 3-diene selective hydrogenation. Journal of Catalysis 2003, 218, 288-295.
28. Kanda Pattamakomsan; EricEhret; FranckMorfin; PatrickGé lin; YvetteJugnet; SwamnyPrakash; Jean ClaudeBertolini; JoongjaiPanpranot; Francisco José Cadete Santos AiresSelective hydrogenation of 1, 3-butadiene over Pd and Pd– Sn catalysts supported on different phases of alumina. Catalysis Today 2011, 164, 28-33.
29. Aurelie Vicente; GwendolineLafaye; CatherineEspecel; PatriceMarecot; Christopher T. Williams The relationship between the structural properties of bimetallic Pd– Sn/SiO2 catalysts and their performance for selective citral hydrogenation. Journal of Catalysis 2011, 283, 133-142.
30. S. K. Johnston; NikolayCherkasov; Elena Pé rez-Barrado; AtteAho; Dmitry Y. Murzin; Alex O. Ibhadon; M. GraziaFrancesconiPd3Sn nanoparticles on TiO2 and ZnO supports as catalysts for semi-hydrogenation: Synthesis and catalytic performance. Applied Catalysis A: General 2017, 544, 40-45.
31. H. Berndt; I. Monnich; B. Lucke; M. MenzelTin promoted palladium catalysts for nitrate removal from drinking water. Applied Catalysis B: Environmental 2001, 30, 111-122.
32. AnthonyGarron; Karoly Lazar; FlorenceEpronEffect of the support on tin distribution in Pd– Sn/Al2O3 and Pd– Sn/SiO2 catalysts for application in water denitration. Applied Catalysis B: Environmental 2005, 59, 57-69.
33. Antonio E. Palomares; CristinaFranch; AvelinoCormaNitrates removal from polluted aquifers using (Sn or Cu)/Pd catalysts in a continuous reactor. Catalysis Today 2010, 149, 348-351.
34. UlfPrьsse; Klaus-DieterVorlopSupported bimetallic palladium catalysts for water-phase nitrate reduction. Journal of Molecular Catalysis A: Chemical 2001, 173, 313-328.
35. Jacinto Sá; Dana Gasparovicova; Konrad Hayek; Erich Halwax; James A. Anderson; Hannelore VinekWater Denitration over a Pd– Sn/Al2O3 Catalyst. Catalysis Letters 2005, 105, 209– 217.
36. AlbinPintar; Jurka Batista; IgorMusevicPalladium-copper and palladium-tin catalysts in the liquid phase nitrate hydrogenation in a batch-recycle reactor. Applied Catalysis B: Environmental 2004, 52, 49-60.
37. Roberto Lanza; Marco Bersani; Luca Conte; Alessandro Martucci; Paolo Canu; Massimo Guglielmi; Giovanni Mattei; Valentina Bello; Massimo Centazzo; Renzo RoseiEffect of Crystalline Phase and Composition on the Catalytic Properties of PdSn Bimetallic Nanoparticles in the PROX Reaction. J. Phys. Chem. C 2014, 118(44), 25392-25402.
38. ZhenyangZhao; BoweiWang; JianMa; WangchengZhan; LiWang; YanglongGuo; YunGuo; GuanzhongLuCatalytic combustion of methane over Pd/SnO2 catalysts. Chinese Journal of Catalysis August 2017, Volume 38, Issue 8, Pages 1322-1329.
39. M. A. Fraga; E. Soares de Souza; F. Villain; L. G. Appel Addition of La and Sn to alumina-supported Pd catalysts for methane combustion. Applied Catalysis A: General 2004, 259, 57-63.
40. W. Lin; L. Lin; Y. X. Zhu; Y. C. Xie; K. Scheurell; E. KemnitzNovel Pd/SnxZr1− xO2 catalysts for methane total oxidation at low temperature and their 18O-isotope exchange behavior. Applied Catalysis B: Environmental 2005, 57, 175-181.
41. Joshua J. Willis; Emmett D. Goodman; Liheng Wu; Andrew R. Riscoe; Pedro Martins; Christopher J. Tassone; MatteoCargnelloSystematic Identification of Promoters for Methane Oxidation Catalysts Using Size- and Composition-Controlled Pd-Based Bimetallic Nanocrystals. J. Am. Chem. Soc. 2017, 139(34), 11989-11997.
42. Juan J. Lovуn-Quintana; Joao B. O. Santos; Adriana S. P. Lovon; Nathalia La-Salvia; Gustavo P. Valenç aLow-temperature oxidation of methane on Pd-Sn/ZrO2 catalysts. Journal of Molecular Catalysis A: Chemical 2016, 411, 117-127.
43. J. Arana; P. Ramirez de la Piscina; J. Llorca; J. Sales; N. Homs; J. L. G. FierroBimetallic Silica-Supported Catalysts Based on Ni− Sn, Pd− Sn, and Pt− Sn as Materials in the CO Oxidation Reaction. Chem. Mater. 1998, 10(5), 1333-1342.
44. D. B. Vasilchenko; R. V. Gulyaev; E. M. Slavinskaya; O. A. Stonkus; Yu V. Shubin; S. V. Korenev; A. I. BoroninEffect of Pd deposition procedure on activity of Pd/Ce0. 5Sn0. 5O2 catalysts for low-temperature CO oxidation. Catalysis Communications 2016, 73, 34-38.
45. T. Yu Kardash; E. M. Slavinskaya; R. V. Gulyaev; A. V. Zaikovskii; S. A. Novopashin; A. I. BoroninEnhanced Thermal Stability of Pd/Ce– Sn– O Catalysts for CO Oxidation Prepared by Plasma-Arc Synthesis. Topics in Catalysis 2017, 60, 898– 913.
46. Cheng Rao; Cheng Peng; Honggen Peng; Li Zhang; Wenming Liu; Xiang Wang; Ning Zhang; Peng WuIn Situ Embedded Pseudo Pd– Sn Solid Solution in Micropores Silica with Remarkable Catalytic Performance for CO and Propane Oxidation. ACS Appl. Mater. Interfaces 2018, 10(11), 9220-9224.
47. Simon J. Freakley; Qian He; Jonathan H. Harrhy; Li Lu, David A. Crole; David J. Morgan; Edwin N. Ntainjua; Jennifer K. Edwards; Albert F. Carley; Albina Y. Borisevich; Christopher J. Kiely; Graham J. Hutchings. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 2016, 351, 965-968.
48. Junbo Zhang; Qi Shao; Ying Zhang; Shuxing Bai; Yonggang Feng; Xiaoqing Huang. Promoting the Direct H2O2 Generation Catalysis by Using Hollow Pd– Sn Intermetallic NanoparticlesSmall 2018, 14(16), 1703990(1)-1703990(7).
49. Neil M. Wilson; David W. FlahertyMechanism for the Direct Synthesis of H2O2 on Pd Clusters: Heterolytic Reaction Pathways at the Liquid– Solid Interface. J. Am. Chem. Soc. 2016, 138(2), 574-586.
50. David W. FlahertyDirect Synthesis of H2O2 from H2 and O2 on Pd Catalysts: Current Understanding, Outstanding Questions, and Research Needs. ACS Catal. 2018, 8(2), 1520-1527.
51. Fumin Li; Qi Shao; Mancheng Hu; Yu Chen; Xiaoqing HuangHollow Pd– Sn Nanocrystals for Efficient Direct H2O2 Synthesis: The Critical Role of Sn on Structure Evolution and Catalytic Performance. ACS Catal. 2018, 8(4), 3418-3423.
52. A. S. Ivanova; E. M. Slavinskaya; R. V. Gulyaev; V. I. Zaikovskii; О. А. Stonkus; I. G. Danilova; L. M. Plyasova; I. A. Polukhina; A. I. BoroninMetal– support interactions in Pt/Al2O3 and Pd/Al2O3 catalysts for CO oxidation. Applied Catalysis B: Environmental 9 June 2010, Volume 97, Issues 1– 2, Pages 57-71.
53. E. M. Slavinskaya; O. A. Stonkus; R. V. Gulyaev; A. S. Ivanova; V. I. Zaikovskii; P. A. Kuznetsov; A. I. BoroninStructural and chemical states of palladium in Pd/Al2O3 catalysts under self-sustained oscillations in reaction of CO oxidation. Applied Catalysis A: General 15 July 2011, Volume 401, Issues 1– 2, Pages 83-97.
54. M. Delage; B. Didillon; Y. Huiban; J. Lynch; D. Uzio. Highly dispersed Pd based catalysts for selective hydrogenation reactions. Studies in Surface Science and Catalysis 2000, Volume 130, Pages 1019-1024.
55. V. Bratan; C. Munteanu; C. Hornoiu; A. Vasile; F. Papa; R. State; S. Preda; D. Culita; N. I. IonescuCO oxidation over Pd supported catalysts — In situ study of the electric and catalytic properties. Applied Catalysis B: Environmental 15 June 2017, Volume 207, Pages 166-173.
56. MarcoD’ Arino; FrancescoPinna; GiorgioStrukulNitrate and nitrite hydrogenation with Pd and Pt/SnO2 catalysts: the effect of the support porosity and the role of carbon dioxide in the control of selectivity. Applied Catalysis B: Environmental 8 November 2004, Volume 53, Issue 3, Pages 161-168.
57.

| © 2019 by the authors. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (http: //creativecommons. org/licenses/by/4. 0/). |
[И Б 1]Applied Catalysis B: Environmental
Volume 53, Issue 3, 8 November 2004, Pages 161-168
|
|
|
© helpiks.su При использовании или копировании материалов прямая ссылка на сайт обязательна.
|