
- Автоматизация
- Антропология
- Археология
- Архитектура
- Биология
- Ботаника
- Бухгалтерия
- Военная наука
- Генетика
- География
- Геология
- Демография
- Деревообработка
- Журналистика
- Зоология
- Изобретательство
- Информатика
- Искусство
- История
- Кинематография
- Компьютеризация
- Косметика
- Кулинария
- Культура
- Лексикология
- Лингвистика
- Литература
- Логика
- Маркетинг
- Математика
- Материаловедение
- Медицина
- Менеджмент
- Металлургия
- Метрология
- Механика
- Музыка
- Науковедение
- Образование
- Охрана Труда
- Педагогика
- Полиграфия
- Политология
- Право
- Предпринимательство
- Приборостроение
- Программирование
- Производство
- Промышленность
- Психология
- Радиосвязь
- Религия
- Риторика
- Социология
- Спорт
- Стандартизация
- Статистика
- Строительство
- Технологии
- Торговля
- Транспорт
- Фармакология
- Физика
- Физиология
- Философия
- Финансы
- Химия
- Хозяйство
- Черчение
- Экология
- Экономика
- Электроника
- Электротехника
- Энергетика
і§б§а§г§а§Т§н §б§а§Э§е§й§Ц§Я§Ъ§с
§і§б§а§г§а§Т§н §б§а§Э§е§й§Ц§Я§Ъ§с
1) §°§Т§л§Ъ§Ы §г§б§а§г§а§Т. §Ј§Щ§С§Ъ§Ю§а§Х§Ц§Ы§г§д§У§Ъ§Ц §Ю§Ц§д§С§Э§Э§С §г §Ф§С§Э§а§Ф§Ц§Я-§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ю§Ъ§е§Ф§Э§Ц§У§а§Х§а§в§а§Х§а§У
§Ў) §®§Ц§д§С§Э§Э§н §г§а §г§в§Ц§Х§Я§Ц§Ы §С§Ь§д§Ъ§У§Я§а§г§д§о§р (Be, Mg, Ca, Sr, Ba, Al, Zn, Li)
§Ј§Щ§С§Ъ§Ю§а§Х§Ц§Ы§г§д§У§е§р§д §г §Ф§С§Э§а§Ф§Ц§Я§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ю§Ъ §У §Т§Ц§Щ§У§а§Х§Я§н§з §Ъ§Я§Ц§в§д§Я§н§з §в§С§г§д§У§а§в§Ъ§д§Ц§Э§с§з §г §а§Т§в§С§Щ§а§У§С§Я§Ъ§Ц§Ю §Ю§Ц§д§С§Э§Э§а§в§Ф§С§Я§Ъ§й§Ц§г§Ь§Ъ§з §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§Ы.
C2H5Br + Mg -> C2H5MgBr
§і6H5Br + Li -> (THF) C6H5Li + LiBr
§ў) §Ї§Ъ§Щ§Ь§С§с §в§Ц§С§Ь§и§Ъ§а§Я§Я§С§с §г§б§а§г§а§Т§Я§а§г§д§о (Hg, Pb, Cd, Ni, Cu etc)
§Ј §а§Т§н§й§Я§н§з §е§г§Э§а§У§Ъ§с§з §Я§Ц §У§Щ§С§Ъ§Ю§а§Х§Ц§Ы§г§д§У§е§р§д §г §Ф§С§Э§а§Ф§Ц§Я-§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ю§Ъ §е§Ф§Э§Ц§У§а§Х§а§в§а§Х§а§У, §а§Х§Я§С§Ь§а §Ъ§з §г§б§Э§С§У§н §г §Я§С§д§в§Ъ§Ц§Ю §У§г§д§е§б§С§р§д §У §в§Ц§С§Ь§и§Ъ§р
C2H5Cl + Pb + Na -> (C2H5)4Pb + NaCl
§Ј) §®§Ц§д§С§Э§Э§н §г §У§н§г§а§Ь§а§Ы §С§Ь§д§Ъ§У§Я§а§г§д§о§р. §Ј§Щ§С§Ъ§Ю§а§Х§Ц§Ы§г§д§У§е§р§д §г §Ф§С§Э§а§Ф§Ц§Я-§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ю§Ъ §г §а§Т§в§С§Щ§а§У§С§Я§Ъ§Ц§Ю §Ю§Ц§д§С§Э§Э§а§в§Ф§С§Я§Ъ§й§Ц§г§Ь§Ъ§з §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§Ы §Ь§С§Ь §б§в§а§Ю§Ц§Ш§е§д§а§й§Я§н§з §б§в§а§Х§е§Ь§д§а§У
§і§Ї3§і§Ї2Br + Na -> CH3CH2CH2CH3 + NaBr + CH3CH2Na
CH2CH2Br + Na -> [CH3CH2Br]- + Na+
[CH3CH2Br]- -> .....
2. §І§Ц§С§Ь§и§Ъ§Ъ §Ъ §в§Ц§С§Ь§д§Ъ§У§н §¤§в§Ъ§Я§о§с§в§С
§§е§й§к§Ц §У§г§Ц§Ф§а §У§г§д§е§б§С§р§д §У §в§Ц§С§Ь§и§Ъ§р §¤§Ў§§°§¤§¦§Ї§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ц §С§Э§Ь§С§Я§а§У
§¦§г§Э§Ъ §У §Т§Ц§Я§Щ§а§Э§о§Я§а§Ю §Ь§а§Э§о§и§Ц §Х§У§С §Ф§С§Э§а§Ф§Ц§Я§а§У§н§з §Щ§С§Ю§Ц§г§д§Ъ§д§Ц§Э§с, §д§а §Ю§С§Ф§Я§Ъ§Ы §б§в§Ъ§д§с§Я§Ц§д§г§с §Ь §д§а§Ю§е, §й§Ц§Ы §в§С§Х§Ъ§е§г §Т§а§Э§о§к§Ц
§Ј §Т§а§Э§Ц§Ц §к§Ъ§в§а§Ь§а§Ю §г§Ю§н§г§Э§Ц §У§г§Ц §г§Ъ§Я§д§Ц§Щ§н §г §е§й§С§г§д§Ъ§Ц§Ю §в§Ц§С§Ь§д§Ъ§У§С §¤§в§Ъ§Я§о§с§в§С §Я§С§Щ§н§У§С§р§д §в§Ц§С§Ь§и§Ъ§с§Ю§Ъ §¤§в§Ъ§Я§о§с§в§С
§®§¦§·§Ў§Ї§Є§©§®
I. §±§Ц§в§Ц§Я§а§г §п§Э§Ц§Ь§д§в§а§Я§С §а§д Mg §Ь R-Hal
R-Hal + Mg ---> [R-Hal]- + Mg+
II. §¶§в§С§Ф§Ю§Ц§Я§д§С§и§Ъ§с §С§Я§Ъ§а§Я-§в§С§Х§Ъ§Ь§С§Э§С
[R-Hal] ---> R* + Hal-
III. §Ґ§Ъ§Ю§Ц§в§Ъ§Щ§С§и§Ъ§с §С§Э§Ь§Ъ§Э§о§Я§н§з §в§С§Х§Ъ§Ь§С§Э§а§У
2R* ---> R-R
R* + Mg ---> R-MgHal
§ґ§С§Ь §Ш§Ц §в§Ц§С§Ф§Ъ§в§е§Ц§д §Ъ §Э§Ъ§д§Ъ§Ы.
§і§б§а§г§а§Т§н §б§а§Э§е§й§Ц§Я§Ъ§с §Х§в§е§Ф§Ъ§з §Ю§Ц§д§С§Э§Э§а§в§Ф§С§Я§Ъ§й§Ц§г§Ь§Ъ§з §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§Ы.
CH3CH2MgCl + CdCl2 ---> (CH3CH2)2Cd + MgCl2
§¤§С§Э§а§Ф§Ц§Я §а§Т§в§С§Щ§е§Ц§д §г§У§с§Щ§о §г §Ь§С§д§Ъ§а§Я§а§Ю §Т§а§Э§Ц§Ц §п§Э§Ц§Ь§д§в§а§б§а§Э§а§Ш§Ъ§д§Ц§Э§о§Я§а§Ф§а §Ю§Ц§д§С§Э§Э§С
C2H5Li + ZnCl2 ---> (C2H5)2Zn + LiCl
CH3SiCl3 + CH3MgCl ---> (CH3)2SiCl2 + MgCl2
4) §ґ§в§С§Я§г§Ю§Ц§д§С§Э§Э§Ъ§в§а§У§С§Я§Ъ§Ц
§ґ.§Ь. §і§Ї-§Ь§Ъ§г§Э§а§д§Я§а§г§д§Ъ §С§в§Ъ§Э§Ф§С§Э§а§Ф§Ц§Я§Ъ§Х§а§У §Т§а§Э§о§к§Ц §і§Ї-§Ь§Ъ§г§Э§а§д§Я§а§г§д§Ъ §С§Ь§Э§Ъ§Э§Ф§С§Э§а§Ф§Ц§Я§Ъ§Х§а§У §Ю§Ц§д§С§Э§Э§а§в§Ф§С§Я§Ъ§й§Ц§г§Ь§Ъ§Ц §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§с §Ю§а§Ф§е§д §б§а§Х§У§Ц§в§Ф§С§д§о§г§с §д§в§С§Я§г§Ю§Ц§д§С§Э§Ъ§в§а§У§С§Я§Ъ§р §б§а§п§д§а§Ю§е §С§д§а§Ю §Ю§Ц§д§С§Э§Э§С, §г§У§с§Щ§С§Я§Я§н§Ы §г §С§Э§Ь§Ъ§Э§о§Я§а§Ы §Ф§в§е§б§б§а§Ы §а§Т§в§С§Щ§е§Ц§д §Я§а§У§е§р §г§У§с§Щ§о §г §С§в§Ъ§Э§о§Я§н§Ю §в§С§Х§Ъ§Ь§С§Э§а§Ю
§±§а§Э§с§в§Я§а§г§д§о §г§У§с§Щ§Ъ §і-§®§Ц §Ю§Ц§Я§с§Ц§д§г§с §У §к§Ъ§в§а§Ь§а§Ю §Ъ§Я§д§Ц§в§У§С§Э§Ц §У §Щ§С§У§Ъ§г§Ъ§Ю§а§г§д§Ъ §а§д §б§а§Э§а§Ш§Ц§Я§Ъ§с §Ю§Ц§д§С§Э§Э§С §У §б§Ц§в§Ъ§а§Х§Ъ§й§Ц§г§Ь§а§Ы §г§Ъ§г§д§Ц§Ю§Ц. §°§Х§Я§С§Ь§а §г§а§Ф§Э§С§г§Я§а §п§Ю§б§Ъ§в§Ъ§й§Ц§г§Ь§а§Ю§е §б§в§С§У§Ъ§Э§е, §Ю§Ц§д§С§Э§Э§а§в§Ф§С§Я§Ъ§й§Ц§г§Ь§Ъ§Ц §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§с §г§й§Ъ§д§С§р§д §Ъ§а§Я§Я§н§Ю§Ъ, §Ц§г§Э§Ъ §в§С§Щ§Я§а§г§д§о §Ю§Ц§Ш§Х§е §Щ§Я§С§й§Ц§Я§Ъ§Ц§Ю §ї§° §С§д§а§Ю§С §е§Ф§Э§Ц§в§а§Х§С §Ъ §Ю§Ц§д§С§Э§Э§С §г§а§г§д§С§У§Э§с§Ц§д §а§д 1,5 §Х§а 1,7
§¦§г§Э§Ъ §С§Э§Ь§Ъ§Э§о§Я§С§с §Ф§в§е§б§б§С §Я§Ц§У§Ц§Э§Ъ§Ь§С, §д§а §У§н§г§а§Ь§Ъ§Ы §Ъ§а§Я§Я§н§Ы §з§С§в§С§Ь§д§Ц§в §і-§®§Ц §г§а§а§Т§л§С§Ц§д §а§в§Ф§С§Я§Ъ§й§Ц§г§Ь§а§Ю§е §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§р §г§У§а§Ы§г§д§У§С §С§Я§С§Э§а§Ф§Ъ§й§Я§н§Ц §Х§Э§с §Я§Ц§а§в§Ф§С§Я§Ъ§й§Ц§г§Ь§Ъ§з §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§Ы.
§Ј §й§С§г§д§Я§а§г§д§Ъ §а§Я§Ъ §Ъ§Ю§Ц§р§д §а§Х§Ъ§Я§С§Ь§а§У§е§р §Ь§в§Ъ§г§д§С§Э§Э§Ъ§й§Ц§г§Ь§е§р §г§д§в§е§Ь§д§е§в§е.
§Ј §а§Т§а§Ъ§з §г§Э§е§й§С§с§з §С§д§а§Ю §Ю§Ц§д§С§Э§Э§С §Ъ§Ю§Ц§р§д §Ь§а§Я§ж§Ъ§Ф§е§в§С§и§Ъ§р §Ъ§Я§Ц§в§д§Я§а§Ф§а §Ф§С§Щ§С.
§і§У§с§Щ§о §С§д§а§Ю§С §е§Ф§Э§Ц§в§а§Х§С §г §Х§в§е§Ф§Ъ§Ю§Ъ §п§Э§Ц§Ь§д§в§а§б§а§Э§а§Ш§Ъ§д§Ц§Э§о§Я§н§Ю§Ъ §Ю§Ц§д§С§Э§Э§С§Ю§Ъ §Ъ §С§д§а§Ю§С§Ю§Ъ, §с§У§Э§с§Ц§д§г§с §б§в§Ц§Ъ§Ю§е§л§Ц§г§д§У§Ц§Я§Я§а §Ь§а§У§С§Э§Ц§Я§д§Я§а§Ы §б§а§Э§с§в§Я§а§Ы, §У §п§д§а§Ю §г§Э§е§й§С§Ц §С§д§а§Ю §Ю§Ц§д§С§Э§Э§С §е§Ш§Ц §Я§Ц §Ъ§Ю§Ц§Ц§д §Ь§а§Я§ж§Ъ§Ф§е§в§С§и§Ъ§р §Ъ§Я§Ц§в§д§Я§а§Ф§а §Ф§С§Щ§С. §Є §г§е§л§Ц§г§д§У§е§р§д §Ь§С§Ь §Х§Ъ§Ю§Ц§в§н §г §д§в§Ч§з§и§Ц§Я§д§в§а§У§н§Ю§Ъ §Х§У§е§з§п§Э§Ц§Ь§д§в§а§Я§Я§н§Ю§Ъ §г§У§с§Щ§с§Ю§Ъ.
§Ґ§Ъ§С§Э§Ь§Ъ§Э§о§Я§н§Ц §б§в§а§Ъ§Щ§У§а§Х§Я§н§Ц §Ю§С§Ф§Я§Ъ§с §в§С§г§г§Ю§С§д§в§Ъ§У§С§р§д §Ь§С§Ь §б§а§Э§Ъ§Ю§Ц§в§н. §ї§Э§Ц§Ь§д§в§а§Я§Я§е§р §Ь§а§Я§ж§Ъ§Ф§е§в§С§и§Ъ§р §Ъ§Я§Ц§в§д§Я§а§Ф§а §Ф§С§Щ§С §Ю§Ц§д§С§Э§Э §Ю§а§Ш§Ц§д §Х§а§г§д§Ъ§Ф§С§д§о §Щ§С §г§й§Ч§д §У§Щ§С§Ъ§Ю§а§Х§Ц§Ы§г§д§У§Ъ§с §г §Ю§а§Э§Ц§Ь§е§Э§С§Ю§Ъ §в§С§г§д§У§а§в§Ъ§д§Ц§Э§с. §Ї§С§б§в§Ъ§Ю§Ц§в §в§Ц§С§Ь§д§Ъ§У §¤§в§Ъ§Я§о§с§в§С §У §в§С§Щ§Т§С§У§Э§Ц§Я§Я§а§Ю §в§С§г§д§У§а§в§Ц §Х§Ъ§п§д§Ъ§Э§а§У§а§Ф§а §п§ж§Ъ§в§С §г§е§л§Ц§г§д§У§е§Ц§д §Ь§С§Ь §Ю§а§Я§а§Ю§Ц§в, §г§а§Ц§Х§Ъ§Я§Ч§Я§Я§н§Ы §г §Х§У§е§Ю§с §Ю§а§Э§Ц§Ь§е§Э§С§Ю§Ъ §Х§Ъ§п§д§Ъ§Э§а§У§а§Ф§а §п§ж§Ъ§в§С.
§·§Ъ§Ю§Ъ§й§Ц§г§Ь§Ъ§Ц §в§Ц§С§Ь§и§Ъ§Ъ
1) §®§°§і-§а§г§Я§а§У§С§Я§Ъ§с (§г§Ъ§Э§о§Я§н§Ц)
2) §®§°§і-§Я§е§Ь§Э§Ц§а§ж§Ъ§Э§о§Я§н§Ц §в§Ц§С§Ф§Ц§Я§д§н
R-MgHal + H-B ---> R-H + MgBHal
CH3MgI + H2O ---> CH4 + Mg(OH)I
CH3MgI + R-OH ---> CH4 + Mg(OR)I
CH3MgI + RCOOH ---> CH4 + RCOOMgI
CH3MgI + NH3 ---> CH4 + Mg(NH2)I
CH3MgI + R-NH2 ---> CH4 + Mg(R-NH2)I
CH3MgI + R-SH ---> CH4 + Mg(R-S)I
CH3MgI + R-CЎФ§і ---> CH4 + R-CЎФ§і-MgI
§®§Ц§д§а§Х §Ь§С§й§Ц§г§д§У§Ц§Я§Я§а§Ф§а §Ъ §Ь§а§Э§Ъ§й§Ц§г§д§У§Ц§Я§Я§а§Ф§а §а§б§в§Ц§Х§Ц§Э§Ц§Я§Ъ§с §Ю§Ц§д§С§Я§С - §Ю§Ц§д§а§Х §№§е§Ф§С§Ц§У§С-§ё§Ц§в§У§Ъ§к§Я§Ъ§Ь§а§У§С - §б§е§д§Ч§Ю §Ъ§Щ§Ю§Ц§в§Ц§Я§Ъ§с §а§Т§м§Ч§Ю§С §У§н§Х§Ц§Э§Ъ§У§к§Ц§Ф§а§г§с §Ю§Ц§д§С§Я§С §б§в§Ъ §У§Щ§С§Ъ§Ю§а§Х§Ц§Ы§г§д§У§Ъ§Ъ §г §а§б§в§Ц§Х§Ц§Э§Ч§Я§Я§н§Ю §Ь§а§Э§Ъ§й§Ц§г§д§У§а§Ю §С§Я§С§Э§Ъ§Щ§Ъ§в§е§Ц§Ю§а§Ф§а §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§с.
§і§Ъ§Я§д§Ц§д§Ъ§й§Ц§г§Ь§а§Ц §б§в§Ъ§Ю§Ц§Я§Ц§Я§Ъ§Ц. §®§Ц§д§а§Х §У§а§г§г§д§С§Я§а§У§Э§Ц§Я§Ъ§с §Ф§С§Э§а§Ф§Ц§Я-§С§Э§Ь§С§Я§а§У §Ъ §Ф§С§Э§а§Ф§Ц§Я-§С§в§Ъ§Э§а§У §Х§а §е§Ф§Э§Ц§У§а§Х§а§в§а§Х§а§У
(CH3)3CBr + Mg --> (CH3)3CMgBr + H2O ---> (CH3)3CH + Mg(OH)Br
§¤§Ў§§°§¤§¦§Ї§±§І§°§Є§©§Ј§°§Ґ§Ї§Ѕ§¦ §µ§¤§§¦§Ј§°§Ґ§°§І§°§Ґ§°§Ј.
R-Hal §а§Т§л§Ц§Ц §а§Т§а§Щ§Я§С§й§Ц§Я§Ъ§Ц.
R - §С§Ь§Ъ§Э§о§Я§н§Ц, §С§в§Ъ§Э§о§Я§н§Ц, §У§Ъ§Я§Ъ§Э§о§Я§н§Ц, §С§Э§Э§Ъ§Э§о§Я§н§Ц, §б§в§а§б§С§в§Ф§Ъ§Э§о§Я§н§Ц, §и§Ъ§Ь§Э§а§С§Э§Ь§Ъ§Э, §Т§Ц§Я§Щ§Ъ§Э.
§Ў§Э§Ь§Ъ§Э, §У§Ъ§Я§Ъ§Э, §С§в§Ъ§Э - Csp3-Hal and C=Csp2-Hal and H5Csp26-Hal
§І§С§Х§Ъ§Ь§С§Э §Ю§а§Ш§Ц§д §Т§н§д§о §б§Ц§в§У§Ъ§й§Я§а§Ф§а/§У§д§а§в§Ъ§й§Я§а§Ф§а/§д§в§Ц§д§Ъ§й§Я§а§Ф§а §г§д§в§а§Ц§Я§Ъ§с.
§Ї§а§Ю§Ц§Я§Ь§Э§С§д§е§в§С.
CH3-CH(Cl)-CH3
2-§з§Э§а§в§б§в§а§б§С§Я (§Є§А§±§Ў§)
§Ъ§Щ§а§б§в§а§б§Ъ§Э§з§Э§а§в§Ъ§Х §Ъ§Э§Ъ §з§Э§а§в§Ъ§г§д§н§Ы §Ъ§Щ§а§б§в§а§б§Ъ§Э (§в§С§и§Ъ§а§Я§С§Э§о§Я§С§с §Я§а§Ю§Ц§Я§Ь§Э§С§д§е§в§С)
§±§а§Э§е§й§Ц§Я§Ъ§Ц.
1. §Ў§Э§Ь§Ъ§Э§Ф§С§Э§а§Ф§Ц§Я§Ъ§Х§н (§з§Э§а§в§Ъ§Х§н, §Т§в§а§Ю§Ъ§Х§н)
§¤§С§Э§а§Ф§Ц§Я§Ъ§в§а§У§С§Я§Ъ§Ц §Я§С §г§У§Ц§д§е §б§в§Ъ §Я§С§Ф§в§Ц§У§С§Я§Ъ§Ъ. (SR §в§Ц§С§Ь§и§Ъ§с)
2. §Ў§Э§Ь§Ъ§Э§ж§д§а§в§Ъ§Х§н
F2+CoF2 = CoF3 (§ж§д§а§в§Ъ§в§е§р§л§Ъ§Ы §С§Ф§Ц§Я§д)
C5H12 + CoF2 = C5F12 (§б§Ц§в§ж§д§а§в§б§Ц§Я§д§С§Я) + §і§аF2
3. §±§а§Э§е§й§Ц§Я§Ъ§Ц §Ъ§Щ §С§Э§Ь§Ц§Я§а§У (§з§Э§а§в §Ъ §Т§в§а§Ю §б§в§а§Ъ§Щ§У§а§Х§Я§н§Ц)
CH2=CH-CH2-CH3 + Br2 = CH2(Br)-CH(Br)-CH2-CH3 (Ae §в§Ц§С§Ь§и§Ъ§с) §У§Ъ§и-§Х§Ъ§Ф§С§Э§а§Ф§Ц§Я§б§в§а§Ъ§Щ§У§а§Х§Я§а§Ц
CH2=CH-CH2-CH3 + HBr = CH2-CH(Br)-CH2-CH3 (Ae)
CH2=CH-CH2-CH3 + NBS = CH2=CH-CH(Br)-CH3
4. §±§а§Э§е§й§Ц§Я§Ъ§Ц §Ъ§Щ §С§Э§Ь§Ъ§Я§а§У.
5. §±§а§Э§е§й§Ц§Я§Ъ§Ц §Ъ§а§Х-§б§в§а§Ъ§Щ§У§а§Х§Я§н§з
R-Cl + NaI = R-I + NaCl
R-COOAg + I2 = R-I + CO2 + AgI
6. §Є§Щ §г§б§Ъ§в§д§а§У (§Ф§С§Э§а§Ф§Ц§Я§Ъ§Х§н §ж§а§г§ж§а§в§С, §з§Э§а§в§Ъ§г§д§н§Ы §д§Ъ§а§Я§Ъ§Э)
7. §±§а§Э§е§й§Ц§Я§Ъ§Ц §С§в§Ъ§Э§Ф§С§Э§а§Ф§Ц§Я§Ъ§Х§а§У.
§±§в§с§Ю§а§Ц §Ф§С§Э§а§Ф§Ц§Я§Ъ§в§а§У§С§Я§Ъ§Ц (SE §в§Ц§С§Ь§и§Ъ§с)
8. §¤§С§Э§а§Ф§Ц§Я§б§в§а§Ъ§Щ§У§а§Х§Я§а§Ц + §С§Ю§Ъ§Х §Ь§С§Э§Ъ§с = §С§Ю§Ъ§Я§б§в§а§Ъ§Щ§У§а§Х§Я§а§Ц
§Ґ§Ъ§С§Щ§а§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ц + §з§Э§а§в§Ъ§Х §Ю§Ц§Х§Ъ 1 §Ъ§Э§Ъ §Ъ§а§Х§Ъ§Х §Ь§С§Э§Ъ§с §Ъ§Э§Ъ §б§Э§С§У§Ъ§Ь§С§а§У§С§с §Ь§Ъ§г§Э§а§д§С §г §Т§а§в§Я§а§Ы §Ь§Ъ§г§Э§а§д§а§Ы = §Ф§С§Э§а§Ф§Ц§Я§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ц §С§Э§Э§Ъ§Э§а§У.
§і§д§в§а§Ц§Я§Ъ§Ц §Ф§С§Э§а§Ф§Ц§Я§б§в§а§Ъ§Щ§У§а§Х§Я§н§з.
§і§У§с§Щ§о §е§Ф§Э§Ц§в§а§Х->§Ф§С§Э§а§Ф§Ц§Я §б§а§Э§с§в§Ъ§Щ§а§У§С§Я§С.
§¶§Ъ§Щ§Ъ§й§Ц§г§Ь§Ъ§Ц §г§У§а§Ы§г§д§У§С.
§Ј §Т§а§Э§о§к§Ъ§Я§г§д§У§Ц §г§У§а§Ч§Ю §п§д§а §б§а§Э§с§в§Я§н§Ц §г§а§Ц§Х§Ъ§Я§Ц§Я§Ъ§с, §а§Х§Я§С§Ь§а §а§Я§Ъ §Я§Ц §в§С§г§д§У§а§в§с§р§д§г§с §У §У§а§Х§Ц, §Ъ§Щ-§Щ§С §а§д§г§е§д§г§д§У§Ъ§с §У§а§Х§а§в§а§Х§Я§н§з §г§У§с§Щ§Ц§Ы §У §Ю§а§Э§Ц§Ь§е§Э§Ц §Ф§С§Э§а§Ф§Ц§Я§б§в§а§Ъ§Щ§У§а§Х§Я§а§Ф§а.
§Ґ§Э§Ъ§Я§н §г§У§с§Щ§Ц§Ы.
C-F 1,56
C-Cl 1,51
C-Br 1,48
C-I 1,29
§Ґ§Ъ§б§а§Э§о§Я§н§Ы §Ю§а§Ю§Ц§Я§д.
CH2Cl2 - 1.6
CHCl3 - 1.03
CCl4 - 0
§·§Ъ§Ю§Ъ§й§Ц§г§Ь§Ъ§Ц §г§У§а§Ы§г§д§У§С §С§Э§Ь§Ъ§Э§Ф§С§Э§а§Ф§Ц§Я§Ъ§Х§а§У.
SN1 (§Ю§а§Я§а§Ю§а§Э§Ц§Ь§е§Э§с§в§Я§а§Ц §Я§е§Ь§Э§Ц§а§ж§Ъ§Э§о§Я§а§Ц §Щ§С§Ю§Ц§л§Ц§Я§Ъ§Ц) and SN2 (§Т§Ъ§Ю§а§Э§Ц§Ь§е§Э§с§в§Я§а§Ц §Я§е§Ь§Э§Ц§а§ж§Ъ§Э§о§Я§а§Ц §Щ§С§Ю§Ц§л§Ц§Я§Ъ§Ц)
C-C-Cl + OH- = C-C-OH + Cl-
§Ї§а §Ф§Ъ§Х§в§а§Ь§г§Ъ§Х-§С§Я§Ъ§а§Я §Ю§а§Ш§Ц§д §г§б§в§а§У§а§и§Ъ§в§а§У§С§д§о §в§Ц§С§Ь§и§Ъ§р §п§Э§Ъ§Ю§Ъ§Я§Ъ§в§а§У§С§Я§Ъ§с
§І§Ц§С§Ь§и§Ъ§Ъ SN §е §Я§С§г§н§л§Ц§Я§Я§а§Ф§а §С§д§а§Ю§С §е§Ф§Э§Ц§в§а§Х§С.
R-X + NaI = R-I + NaX
R-X + NaOH (H2O) = R-OH + NaX
R-X + R-ONa = R-O-R + NaX
R-X + NH3 = R-NH2 + HX
R-X + NaCN = R-CN + NaX
R-X + R-COONa = R-COO-R + NaX
R-X + HC==CNa = R-C==C-R
R-X + AgNO2 = R-NO2 + AgX
§®§Ц§з§С§Я§Ъ§Щ§Ю §в§Ц§С§Ь§и§Ъ§Ъ §Я§е§Ь§Э§Ц§а§ж§Ъ§Э§о§Я§а§Ф§а §Щ§С§Ю§Ц§л§Ц§Я§Ъ§с §е §Я§С§г§н§л§Ц§Я§Я§а§Ф§а §С§д§а§Ю§С §е§Ф§Э§Ц§в§а§Х§С.
| SN1 | SN2 |
| §Б§У§Э§с§Ц§д§г§с §Х§У§е§з§г§д§С§Х§Ъ§Ы§Я§н§Ю 1. §Ґ§Ъ§г§г§а§и§Ъ§С§и§Ъ§с (§а§Т§в§С§Щ§а§У§С§Я§Ъ§Ц §Ь§С§в§Т§Ь§С§д§Ъ§а§Я§С §Ъ §Ф§С§Э§а§Ф§Ц§Я§Ъ§Х-§С§Я§Ъ§а§Я§С) - §п§д§а §а§Т§в§С§д§Ъ§Ю§н§Ы §б§в§а§и§Ц§г§г 2. §Ї§е§Ь§Э§Ц§а§ж§Ъ§Э§о§Я§С§с §С§д§С§Ь§С. §Ј§Щ§С§Ъ§Ю§а§Х§Ц§Ы§г§д§У§Ъ§Ц §Ь§С§в§Т§Ь§С§д§Ъ§а§Я§С §г §Я§е§Ь§Э§Ц§а§ж§Ъ§Э§а§Ю | §°§Х§Я§а§г§д§С§Х§Ъ§Ы§Я§н§Ы §б§в§а§и§Ц§г§г 1. §Ј§Щ§С§Ъ§Ю§а§Х§Ц§Ы§г§д§У§Ъ§Ц §г §Я§е§Ь§Э§Ц§а§ж§Ъ§Э§а§Ю |
§і§±§Є§І§ґ§Ѕ
§±§в§а§Т§С §§е§Ь§С§г§С (§Ъ§Х§Ц§Я§д§Ъ§ж§Ъ§Ь§С§и§Ъ§с §г§д§в§е§Ь§д§е§в§н §г§б§Ъ§в§д§С)
§І§Ц§С§Ь§д§Ъ§У §Э§е§Ь§С§г§С - HCl + ZnCl2
1) §±§Ц§в§У§Ъ§й§Я§н§Ц §г§б§Ъ§в§д§н §Я§Ц §в§Ц§С§Ф§Ъ§в§е§р§д
2) §Ј§д§а§в§Ъ§й§Я§н§Ц §г§б§Ъ§в§д§н §а§Т§в§С§Щ§е§р§д §з§Э§а§в§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ц (§Я§Ъ§Ш§Я§с§с §ж§С§Щ§С), §Я§С §в§Ц§С§Ь§и§Ъ§р §д§в§Ц§Т§е§Ц§д§г§с §Я§Ц§Ь§а§д§а§в§а§Ц §У§в§Ц§Ю§с
3) §ґ§в§Ц§д§Ъ§й§Я§н§Ц §г§б§Ъ§в§д§н §в§Ц§С§Ф§Ъ§в§е§р§д §Ю§Ф§Я§а§У§Ц§Я§Я§а.
§І§Ц§С§Ь§и§Ъ§Ъ §б§в§а§д§Ц§Ь§С§р§д §б§а SN1 §Ю§Ц§з§С§Я§Ъ§Щ§Ю§е, §Ь§а§д§а§в§н§Ы §Т§а§Э§Ц§Ц §б§в§Ц§Х§б§а§й§д§Ъ§д§Ц§Э§Ц§Я §Х§Э§с §д§в§Ц§д§Ъ§й§Я§н§з §г§д§в§е§Ь§д§е§в.
§±§а§Э§е§й§Ц§Я§Ъ§Ц §Ф§С§Э§а§Ф§Ц§Я-§Щ§С§Ю§Ц§л§Ч§Я§Я§н§з §Ъ§Щ §г§б§Ъ§в§д§а§У §г§е§Э§о§ж§С§Я§С§д§Я§а§Ы §Ф§в§е§б§б§а§Ы
§І§Ц§С§Ь§и§Ъ§Ъ §Х§Ц§Ф§Ъ§Х§в§С§д§С§и§Ъ§Ъ.
1) §Ј§Я§е§д§в§Ъ§Ю§а§Э§Ц§Ь§е§Э§с§в§Я§С§с (§а§Т§в§С§Щ§а§У§С§Я§Ъ§Ц §Ь§в§С§д§Я§н§з §г§У§с§Щ§Ц§Ы)
2) §®§Ц§Ш§Ю§а§Э§Ц§Ь§е§Э§с§в§Я§С§с (§а§Т§в§С§Щ§а§У§С§Я§Ъ§Ц §п§ж§Ъ§в§а§У)
§±§Ц§в§У§Ъ§й§Я§н§Ц §г§б§Ъ§в§д§н §а§Ь§Ъ§г§Э§с§р§д§г§с §Э§Ц§Ф§й§Ц §У§г§Ц§Ф§а. §Ј§д§а§в§Ъ§й§Я§н§Ц §г§Э§а§Ш§Я§Ц§Ц. §±§в§Ъ §а§Ь§Ъ§г§Э§Ц§Я§Ъ§Ъ §д§в§Ц§д§Ъ§й§Я§н§з §б§в§а§Ъ§г§з§а§Х§Ъ§д §Х§Ц§г§д§в§е§Ь§и§Ъ§с §е§Ф§Э§Ц§в§а§Х§Я§а§Ф§а §г§Ь§Ц§Э§Ц§д§С.
§®§Я§а§Ф§а§С§д§а§Ю§Я§н§Ц §г§б§Ъ§в§д§н.
§§Ъ§г§Э§а§д§Я§а§г§д§о §Ю§Я§а§Ф§а§С§д§а§Ю§Я§н§з §г§б§Ъ§в§д§а§У §е§У§Ц§Э§Ъ§й§Ъ§У§С§Ц§д§г§с. §Є§Щ-§Щ§С §б§а§с§У§Э§Ц§Я§Ъ§с §Х§а§б§а§Э§Я§Ъ§д§Ц§Э§о§Я§а§Ф§а §и§Ц§Я§д§в§С §Ь§Ъ§г§Э§а§д§Я§а§г§д§Ъ §Ъ §У§д§а§в§С§с §Ф§в§е§б§б§С §б§а §а§д§Я§а§к§Ц§Я§Ъ§р §Ь §б§Ц§в§У§а§Ы §с§У§Э§с§Ц§д§г§с §п§Э§Ц§Ь§д§в§а§Я-§С§Ь§и§Ц§б§д§а§в§а§Ю, §й§д§а §д§С§Ь §Ш§Ц §б§а§У§н§к§С§Ц§д §Ь§Ъ§г§Э§а§д§Я§н§Ц §г§У§а§Ы§г§д§У§С.
§¶§¦§Ї§°§§Ѕ
§І§Ц§Щ§а§Я§С§Я§г§Я§н§Ц §г§д§в§е§Ь§д§е§в§н §ж§Ц§Я§а§Э§С
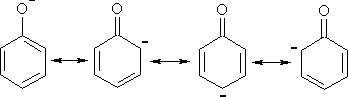
§©§С§Ю§Ц§л§Ц§Я§Ъ§Ц -§°§Ї §Ф§в§е§б§б§н §У §ж§Ц§Я§а§Э§С§з §Я§Ц§У§а§Щ§Ю§а§Ш§Я§а §Ъ§Щ-§Щ§С §г§Ъ§Э§о§Я§а§Ы §г§У§с§Щ§Ъ §і-§° §У §г§Э§Ц§Х§г§д§У§Ъ§Ц +M §п§ж§ж§Ц§Ь§д§С §Ф§Ъ§Х§в§а§Ь§г§Ъ§Э§о§Я§а§Ы §Ф§в§е§б§б§н.
§ґ§в§Ъ§У§Ъ§С§Э§о§Я§а§Ц §Я§С§Щ§У§С§Я§Ъ§Ц §ж§Ц§Я§а§Э§С - §Ь§С§в§Т§а§Э§а§У§С§с §Ь§Ъ§г§Э§а§д§С, §Ь§С§в§Т§а§Э§Ь§С.
§і§Ъ§г§д§Ц§Ю§С§д§Ъ§й§Ц§г§Ь§а§Ц §Я§С§Щ§У§С§Я§Ъ§Ц - §Ф§Ъ§Х§в§а§Ь§г§а§Т§Ц§Я§Щ§а§Э.
§©§С§Ю§Ц§л§Ч§Я§Я§н§Ц (§Ю§Ц§д§Ъ§Э§о§Я§а§Ы §Ф§в§е§б§б§а§Ы) §ж§Ц§Я§а§Э§н §Я§С§Щ§н§У§С§р§д§г§с §§І§¦§©§°§§Ѕ.
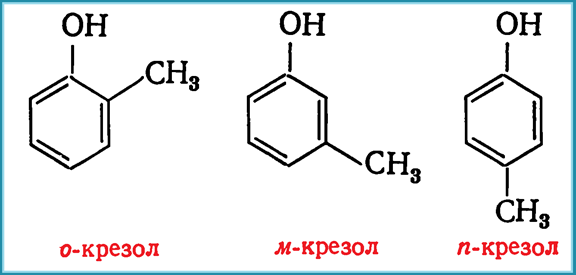
§®§Ї§°§¤§°§Ў§ґ§°§®§Ї§Ѕ§¦ §¶§¦§Ї§°§§Ѕ
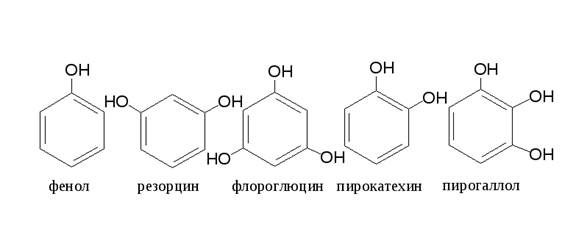
§°§Т§л§Ъ§Ц §ж§Ъ§Щ§Ъ§й§Ц§г§Ь§Ъ§Ц §г§У§а§Ы§г§д§У§С.
-§¶§Ц§Я§а§Э
§ґ§У§Ч§в§Х§а§Ц §У§Ц§л§Ц§г§д§У§а, §й§С§г§д§Ъ§й§Я§а §в§С§г§д§У§а§в§Ъ§Ю§а §У §У§а§Х§Ц (§У §Ф§а§в§с§й§Ц§Ы §У§а§Х§Ц §в§С§г§д§У§а§в§Ъ§Ю§а§г§д§о §е§У§Ц§Э§Ъ§й§Ъ§У§С§Ц§д§г§с). §§в§Ъ§г§д§С§Э§Э§н §в§а§Щ§а§У§а§Ф§а §и§У§Ц§д§С (§Ь§в§С§г§Я§Ц§р§д §Я§С §У§а§Щ§Х§е§з§Ц §Ъ§Щ-§Щ§С §а§Ь§Ъ§г§Э§Ц§Я§Ъ§с).
§і§±§°§і§°§ў§Ѕ §±§°§§µ§№§¦§Ї§Є§Б
§Ј§н§Х§Ц§Э§Ц§Я§Ъ§Ц §Ъ§Щ §Ь§С§Ю§Ц§Я§Я§а§е§Ф§а§Э§о§Я§а§Ы §г§Ю§а§Э§н.
§і§Ъ§Я§д§Ц§д§Ъ§й§Ц§г§Ь§Ъ§Ц §г§б§а§г§а§Т§н §б§а§Э§е§й§Ц§Я§Ъ§с (§г§е§Э§о§ж§Ъ§Я§Ъ§в§а§У§С§Я§Ъ§Ц §Т§Ц§Я§Щ§а§Э§С §Ъ §Щ§С§Ю§Ц§л§Ц§Я§Ъ§Ц §г§е§Э§о§ж§а-§Ф§в§е§б§б§н §Я§С -§°§Ї; §Э§Ъ§Т§а §й§Ц§в§Ц§Щ §Ф§С§Э§а§Ф§Ц§Я-§б§в§а§Ъ§Щ§У§а§Х§Я§н§Ц)
§§е§Ю§а§Э§о§Я§н§Ы §г§б§а§г§а§Т §б§а§Э§е§й§Ц§Я§Ъ§с.
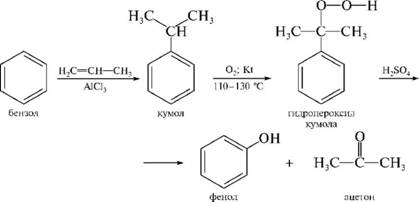
§№§Ц§в§Ц§Щ §в§Ц§С§Ь§и§Ъ§р §Х§Ъ§С§Щ§а§д§Ъ§в§а§У§С§Я§Ъ§с §С§Я§Ъ§Э§Ъ§Я§С §г §б§а§г§Э§Ц§Х§е§р§л§Ъ§Ю §Ф§Ъ§Х§в§а§Э§Ъ§Щ§а§Ю.
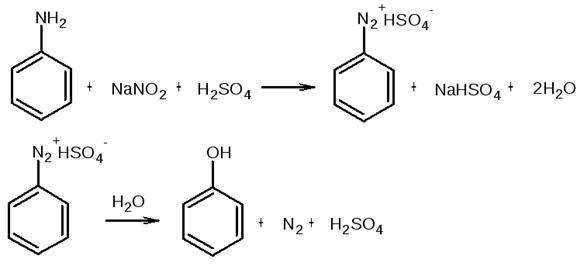
§°§ў§»§Є§¦ §·§Є§®§Є§№§¦§і§§Є§¦ §і§Ј§°§«§і§ґ§Ј§Ў (§Я§С §б§в§Ъ§Ю§Ц§в§Ц §г§С§Ю§а§Ф§а §ж§Ц§Я§а§Э§С)
-§¶§Ц§Я§а§Э §Х§Ъ§г§а§и§Ъ§Ъ§в§е§Ц§д §У §У§а§Х§Ц §г §а§Т§в§С§Щ§а§У§С§Я§Ъ§Ц§Ю §Ъ§а§Я§С §Ф§Ъ§Х§в§а§Ь§г§а§Я§Ъ§с.
-§І§Ц§С§Ф§Ъ§в§е§Ц§д §г §л§Ц§Э§а§й§С§Ю§Ъ §а§Т§в§С§Щ§е§с §ж§Ц§Я§а§Э§с§д§н
/§б§а §Ь§Ъ§г§Э§а§д§Я§н§Ю §г§У§а§Ы§г§д§У§С§Ю §ж§Ц§Я§а§Э §г§Э§С§Т§Ц§Ц §е§Ф§а§Э§о§Я§а§Ы §Ъ §Ь§С§в§Т§а§Я§а§У§н§з §Ь§Ъ§г§Э§а§д/
§Ј§У§Ц§Х§Ц§Я§Ъ§Ц §п§Э§Ц§Ь§д§в§а§Я-§С§Ь§и§Ц§б§д§а§в§Я§н§з §Щ§С§Ю§Ц§г§д§Ъ§д§Ц§Э§Ц§Ы §б§а§У§н§к§С§Ц§д §Ь§Ъ§г§Э§а§д§Я§а§г§д§о §ж§Ц§Я§а§Э§С. §Ї§С§б§в§Ъ§Ю§Ц§в §Ь§Ъ§г§Э§а§д§Я§а§г§д§о §б§Ъ§Ь§в§Ъ§Я§а§У§а§Ы §Ь§Ъ§г§Э§а§д§н §б§в§Ъ§Т§Э§Ъ§Ш§С§Ц§д§г§с §Ь §Ь§а§Я§г§д§С§Я§д§Ц §Х§Ъ§г§г§а§и§Ъ§С§и§Ъ§Ъ §г§а§Э§с§Я§а§Ы §Ь§Ъ§г§Э§а§д§н.
§ї§Э§Ц§Ь§д§в§а§Я-§Х§а§Я§а§в§Я§н§Ц §Ф§в§е§б§б§н §б§а§Я§Ъ§Ш§С§р§д §Ь§Ъ§г§Э§а§д§Я§а§г§д§о.
§©§С§Ю§Ц§л§Ц§Я§Ъ§Ц -§°§Ї §Ф§в§е§б§б§н §Я§Ц§У§а§Щ§Ю§а§Ш§Я§а, §Я§а §ж§Ц§Я§а§Э§н §Ю§а§Ф§е§д §а§Т§в§С§Щ§а§У§н§У§С§д§о §п§ж§Ъ§в§н §б§а §Ф§Ъ§Х§в§а§Ь§г§Ъ§Э§о§Я§а§Ы §Ф§в§е§б§б§Ц.
|
|
|
© helpiks.su При использовании или копировании материалов прямая ссылка на сайт обязательна.
|