
- Автоматизация
- Антропология
- Археология
- Архитектура
- Биология
- Ботаника
- Бухгалтерия
- Военная наука
- Генетика
- География
- Геология
- Демография
- Деревообработка
- Журналистика
- Зоология
- Изобретательство
- Информатика
- Искусство
- История
- Кинематография
- Компьютеризация
- Косметика
- Кулинария
- Культура
- Лексикология
- Лингвистика
- Литература
- Логика
- Маркетинг
- Математика
- Материаловедение
- Медицина
- Менеджмент
- Металлургия
- Метрология
- Механика
- Музыка
- Науковедение
- Образование
- Охрана Труда
- Педагогика
- Полиграфия
- Политология
- Право
- Предпринимательство
- Приборостроение
- Программирование
- Производство
- Промышленность
- Психология
- Радиосвязь
- Религия
- Риторика
- Социология
- Спорт
- Стандартизация
- Статистика
- Строительство
- Технологии
- Торговля
- Транспорт
- Фармакология
- Физика
- Физиология
- Философия
- Финансы
- Химия
- Хозяйство
- Черчение
- Экология
- Экономика
- Электроника
- Электротехника
- Энергетика
Risk controls
Human tissue and body fluids
Sources of exposure/industries
Hazards associated with needlestick injury
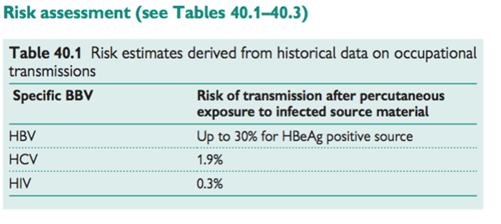
- Main hazards: HBV, HCV, HIV (b p. 152, Hepatitis B; b p. 154, Hepatitis C; b p. 156, Human immunodeficiency virus)
- Any blood-borne infection (e.g. malaria) can be transmitted by needlestick injury (NSI)
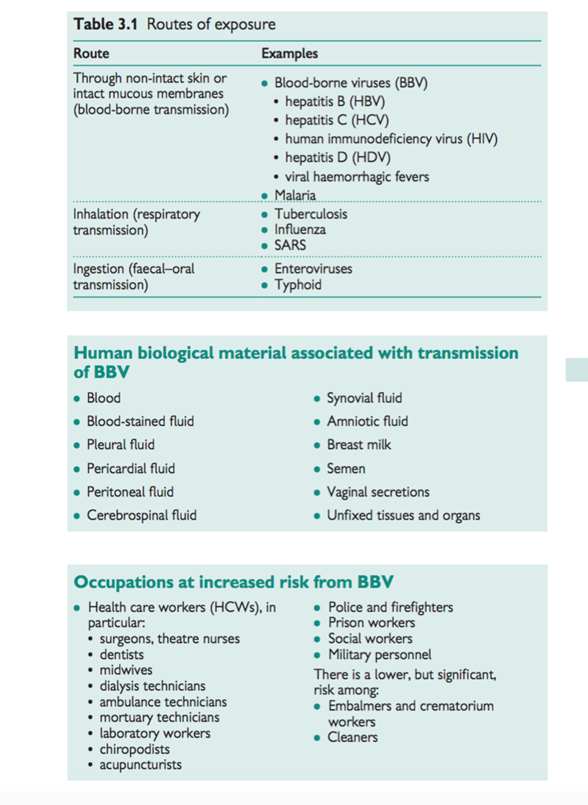
See Table 3.1 for routes of exposure.
Respiratory infections
Those who undertake aerosol-generating procedures, e.g. post-mortem staff, physiotherapists (suction and expectoration), bronchoscopy staff.
Faecal–oral infections
Sewage workers, laboratory staff.
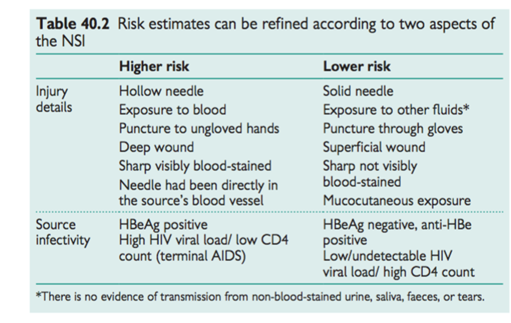
Factors affecting exposure and risk assessment
The risk of transmission is determined by:
• Dose or level of exposure depends on the details of the incident including route of exposure and body fluid involved
• Source infectivity.
Risk controls
• Adherence to standard infection control procedures, including hand hygiene, use of PPE (gloves for procedures that involve a risk of contamination, double gloves for surgical procedures on patients known to be infected with BBV). Aprons, goggles, and mask are required where there is a risk of splashing, boots or overshoes if floor is contaminated. Other risk controls include:
- use of safer sharps devices, avoidance of re-sheathing needles
- correct disposal of sharps and infected waste
- correct transport of specimens
- filtering respiratory masks for aerosol-generating procedures
- immunization against HBV, TB, influenza
- appropriate decontamination procedures for spills
- Prompt management of sharps and contamination incidents
Management of needlestick and contamination incidents
Hazards associated with needlestick injury
- Main hazards: HBV, HCV, HIV
- Any blood-borne infection (e.g. malaria) can be transmitted by needlestick injury (NSI)
- Classification of contamination incidents
- Percutaneous: when a contaminated sharp breaches intact skin
- Mucocutaneous: when blood or body fluids splash onto mucous
membranes or non-intact skin
- 2 Intact normal skin is an effective barrier against BBV.
Immediate first aid
• Wash wound with soap and water; encourage bleeding gently • Irrigate exposed mucous membranes copiously with water.
Source testing
Source patients should generally be tested for HBV, HCV, and HIV:
- Pre-test discussion and informed consent: are essential, and may be
carried out by any appropriately trained and competent health care worker
- The unconscious source patient: should not normally be tested until
consent has been obtained. If necessary, PEP should be commenced until the patient awakes. If a source patient has died, consent should normally be obtained from a relative.

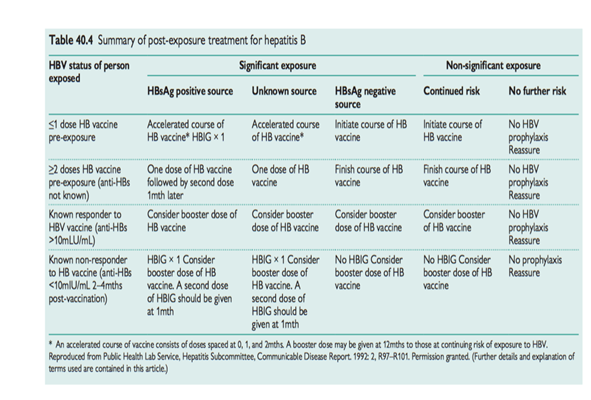
Management of needlestick and contamination incidents 2: hepatitis B post-exposure prophylaxis
Indications
Significant occupational exposure to HBV positive source material.
Regime
- Treatment depends on whether the recipient has been immunized against HBV and (if so) whether they have achieved adequate immunity. See Table 40.4.
- Hepatitis B specific immunoglobulin (HBIG) is usually provided by the local HPA laboratory. If you provide cover for managing NSIs, ensure that clear arrangements are in place to access HBIG promptly if indicated.
Management of needlestick and contamination incidents 3: human immunodeficiency virus post-exposure prophylaxis
Indications. Significant occupational exposure to source material that is known to be infected with HIV, or high risk of infection and HIV test is not obtainable.
Drug regime . A combination of at least three oral anti-retroviral agents for 4wks, includ- ing both nucleoside analogue reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs).

Timing of PEP
The EAGA recommends that PEP is given as soon as possible after expo- sure and certainly within 48–72h of exposure, and continued for at least 28 days. It is not generally recommended to commence PEP beyond 72h post-exposure, but this is a matter for the judgement of local experienced clinicians.
Side effects of PEP
- Serious side effects are rare, but one death has been reported with a previous PEP regime for an occupational exposure
- Unpleasant minor side effects (GI upset, headache) common; treatment with adjuvant anti-emetics and anti-diarrhoeals often required
- The newer PEP regime generally better tolerated than previous combination therapy, but there is still a high incidence of failure to adhere.
Efficacy of PEP
- Advice on HIV PEP is based on indirect evidence of efficacy in the prevention of vertical transmission of HIV, and on surveillance data following occupational exposures
- PEP with Zidovudine has been shown (in a case control study) to reduce the risk of occupational transmission by 80%
- There have been documented cases of occupational transmission of HIV despite appropriate PEP.
Microbial pathogens (in laboratory settings)
Common sources . Exposure to dangerous pathogens through work occurs almost exclusively in the experimental or clinical laboratory setting, often in health care or veterinary science (see Table 3.2).
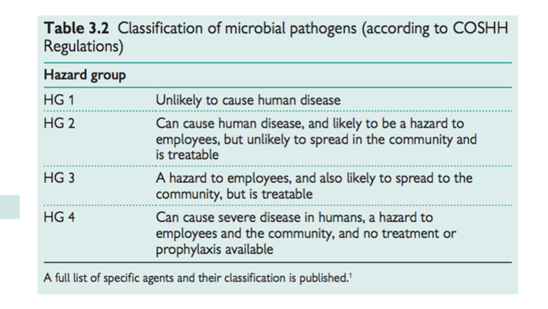
Factors that affect the risk assessment
• Consequence of infection (serious human disease) • Potential for transmission:
- infect and harm employees
- spread to the community • Amenability to treatment.
Exposure controls
• Containment: three levels of containment for Hazard Group 2–4 pathogens, including:
- separation from other activities
- –ve pressure ventilation
- high-efficiency particulate absorption (HEPA) filtered air intake and
output
- restriction to authorized personnel (e.g. access controls)
- safety cabinet
- observation window to allow monitoring from outside.
Use of PPE including respiratory protective equipment
- Emergency/incident planning (handling accidents)
- Vector control (rats mainly)
- Display biohazard warnings
- Safe decontamination and disinfection procedures
- Safe waste management
- Safe transport of pathogens
- Good hygiene: separation of eating areas for staff, hand washing routines.
Occupational health input
- Immunization where available
- Health surveillance: in practice this consists mainly of education to be vigilant and report symptoms, record of immunity
- Advise on individual susceptibility, e.g. pregnancy, immunosuppression.
Animals and animal products
Common sources and industries
Any industry that involves direct contact with animals (live or dead), their excreta, or products:
• Agriculture
• Veterinary medicine
• Meat processing (including abbatoirs), packing, and distribution.
Potential health effects
Zoonoses
These are a group of infections typically found in animals as the primary host, but which spread from animals to humans (see Table 3.3). Some can be transmitted from human to human. There are approximately 40 poten- tial zoonoses in the UK and approximately 300,000 people in a variety of occupations are potentially exposed. Although most zoonoses are mild and self-limiting, some may cause long-term health effects.
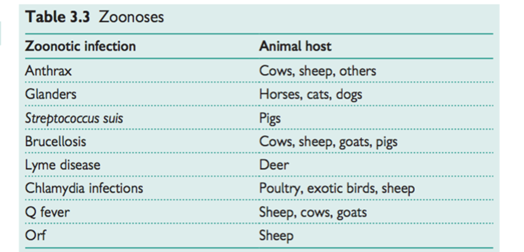
Allergic (immune-mediated) disease
Some organic antigens are animal products (e.g. rat urine), or found in association with animal products (e.g. bloom on bird feathers)
Risk assessment
• Route of exposure: high risk with skin contamination, inhalation of dusts and aerosols, and ingestion.
Prevention/exposure control
• Good husbandry practices for livestock:
- good standards of hygiene in young-stock housing
- low stocking densities
- avoid contaminating animal drinking water with dung
- keep animals as stress-free as possible
• Education and awareness of zoonoses:
- warn employees and visitors about the risk of zoonoses and preventive measures
- advise early consultation with a doctor and declaration of exposure to animals if suspicious symptoms occur
• Identify those with individual susceptibility and restrict from exposure:
- pregnant women (avoid pregnant sheep)
- immune compromised people
• Immunizing and treating livestock
• Good occupational hygiene practices (Table 3.4).
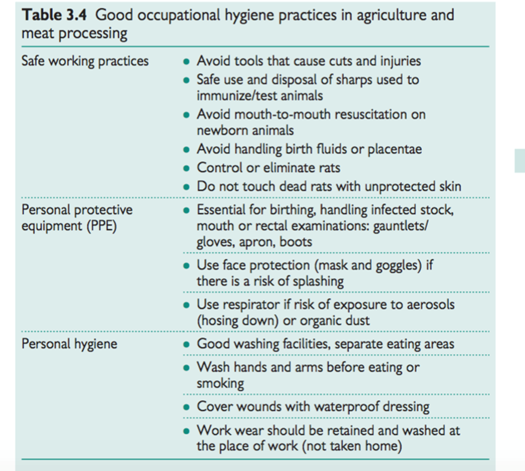
Organic dusts and mists
These are a group of biological agents that have the potential to cause occupational disease, and are widespread in the workplace They are mainly high molecular weight proteins from plant and animal material and micro-organisms.
Common sources
Organic dusts
• Animal proteins:
urine and dander from farm or laboratory animals (e.g. cows, rats)
• Plant proteins:
- natural rubber latex
- grain dust
- flourdust
- wood dusts
- colophony
• Microbial:
- moulds and spores that grow in vegetable matter (e.g. hay,
mushroom compost)
- enzymes.
Organic mists
- Proteinaceous mists from washing fish products, and surfaces or equipment contaminated with fish/animal proteins
- Bacterially infected metalworking fluids.
Specific industries
• Health care industry
• Rubber manufacturing
• Laboratories and animal houses/care facilities • Farming
• Baking and flour milling
• Biological detergent manufacture
• Fish processing
• Engineering.
Health effects
• Type I allergy (IgE-mediated):
- occupational asthma
- allergic rhinitis
- contact urticaria
- anaphylaxis
• Hypersensitivity pneumonitis.
Factors affecting the risk assessment
- Exposure
- Potency of the specific allergen
- Individual susceptibility (e.g. atopy, previous sensitization,
cross-reactivity to similar allergens).
Risk controls
- Minimize exposure: generic principles
- good animal husbandry, including avoidance of overcrowding
- good hygiene—regular cleaning of animal cages and housing, wood
workshops, bakeries
- general and local ventilation
- dust abatement techniques: avoid dry sweeping or compressed air
lines for cleaning; instead use an industrial vacuum cleaner or wet
clean
- Detailed guidance on the following specific biological allergens is
available at M http://www.hse.gov.uk/asthma/index.htm
- flourdust
- grain dust
- laboratory animals
- natural rubber latex
- wood dust.
- Use of PPE: can be used if a significant risk exists after appropriate
efforts at exposure control, e.g. for intermittent dusty tasks.
H Some advocate the use of respiratory protective equipment (RPE) as a last resort in sensitized workers whose livelihood depends on working in ‘at-risk’ situations (e.g. farmers). If this approach is advised, it must be with extreme caution, and then only after all possible efforts have been made to reduce exposure. The individual must be monitored closely (health surveillance) for signs of deterioration.
Health surveillance
All those who are exposed to a significant risk of allergic disease must have health surveillance as required by the Management of Health and Safety at Work Regulations.
- Regular symptoms questionnaire and lung function
- Follow-up positive symptoms with further investigation:
- serial peak flow tests
- skin prick tests
- skin patch tests
- total IgE and specific IgE for suspect agent (e.g. latex).
- Exclude if exposure cannot be controlled adequately, or use PPE and monitor extremely closely.
|
|
|
© helpiks.su При использовании или копировании материалов прямая ссылка на сайт обязательна.
|